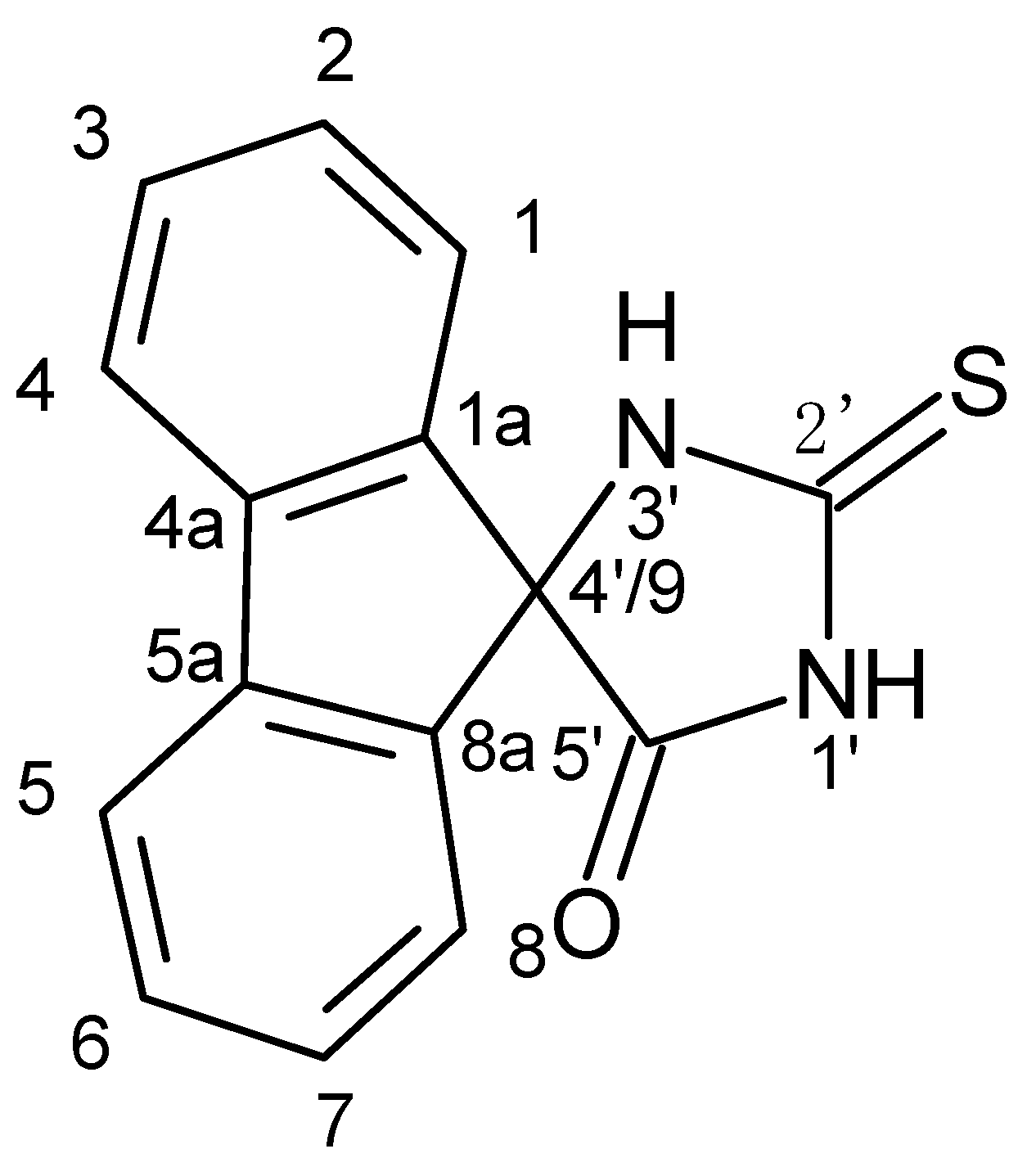
5′-Oxospiro-(fluorene-9,4′-imidazolidine)-2′-thione
Abstract
1. Introduction
2. Results
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, W.; Miller, M.J. Concise Synthesis of 4-Acylamino Analogues of 2-Aminobicyclo[3.1.0]hexane-2,6-dicarboxylic Acids (LY354740) from an Acylnitroso Diels-Alder Cycloadduct. J. Org. Chem. 2004, 69, 4516–4519. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, K.; Kumagai, T.; Taguchi, T.; Nakazato, A. Scalable Synthesis of (+)-2-Amino-3-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic Acid as a Potent and Selective Group II Metabotropic Glutamate Receptor Agonist. Chem. Pharm. Bull. 2007, 55, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Oishi, S.; Kang, S.-U.; Liu, H.; Zhang, M.; Yang, D.; Deschamps, J.R.; Burke, T.R. Synthesis of α,α-Disubstituted 4-Phosphonophenylalanine Analogs as Conformationally-constrained Phosphotyrosyl Mimetics. Tetrahedron 2004, 60, 2971–2977. [Google Scholar] [CrossRef]
- Martins, F.J.C.; van der Hoven, H.; Viljoen, A.M. Synthesis of Exo-3-amino-10-hydroxy-hexacyclo[10.2.1.02,11.04,10.04,14.09,13]-pentadecane-5,7-diene-endo-3-carboxyclic Acid and Endo-3-amino-10-hydroxy-hexacyclo[10.2.1.02,11.04,10.04,14.09,13]pentadecane-5,7-dieneexo-3-carboxylic Acid. Tetrahedron 2009, 65, 2921–2926. [Google Scholar] [CrossRef]
- Goebel, T.; Ulmer, D.; Projahn, H.; Kloeckner, J.; Heller, E.; Glaser, M.; Ponte-Sucre, A.; Specht, S.; Sarite, S.R.; Hoerauf, A.; et al. In Search of Novel Agents for Therapy of Tropical Diseases and Human Immunodeficiency Virus. J. Med. Chem. 2008, 51, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lou, J.; Wang, Z.; Wang, T.; Xiao, Y.; Hu, X.; Liu, P.; Hong, X. Design, Synthesis and Pharmacological Evaluation of Novel N-(2-(1,1-Dimethyl-5,7-Dioxo-4,6-diazaspiro[2.4]heptan-6-yl)ethyl) Sulfonamide Derivatives as Potential Anticonvulsant Agents. Eur. J. Med. Chem. 2015, 92, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Pan, Y.; Xu, Z.; Li, R.; Qiu, G.; Xu, W.; Ke, X.; Wu, L.; Hu, X. Synthesis and Potential Anticonvulsant Activity of New N-3- Substituted 5,5-Cyclopropanespirohydantoins. Eur. J. Med. Chem. 2009, 44, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Suzuki, M.; Kanai, M.; Shibasaki, M. General and Practical Catalytic Enantioselective Strecker Reaction of Keto-imines: Significant Improvement through Catalyst Tuning by Protic Additives. Tetrahedron Lett. 2004, 45, 3147–3151. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Zhou, J. Organocatalytic Asymmetric Cyanation of Isatin Derived N-Boc ketoimines. Chem. Commun. 2013, 49, 4421–4423. [Google Scholar] [CrossRef] [PubMed]
- Bakalova, A.; Buyukliev, R.; Momekov, G. Synthesis, DFT Calculations and Cytotoxic Investigation of Platinum Complexes with 3-Thiolanespiro-5′-hydantoin and 4-Thio-1H-tetrahydropyranespiro-5′-hydantoin. J. Mol. Struct. 2015, 1091, 118–124. [Google Scholar] [CrossRef]
- Marinova, P.; Marinov, M.; Kazakova, M.; Feodorova, Y.; Penchev, P.; Sarafian, V.; Stoyanov, N. Synthesis and in vitro activity of platinum(II) complexes of two fluorenylspirohydantoins against a human tumour cell line. Biotechnol. Biotechnol. Equip. 2014, 28, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Ahmedova, A.; Marinova, P.; Marinov, M.; Stoyanov, N. An integrated experimental and quantum chemical study on the complexation properties of (9′-fluorene)-spiro-5-hydantoin and its thioanalogue. J. Mol. Struct. 2016, 1108, 602–610. [Google Scholar] [CrossRef]
- Marinova, P.; Marinov, M.; Kazakova, M.; Feodorova, Y.; Blazheva, D.; Slavchev, A.; Sbirkova-Dimitrova, H.; Sarafian, V.; Stoyanov, N. Crystal Structure of 5′-Oxospiro-(fluorene-9,4′-imidazolidine)-2′-thione and Biological Activity of Its Derivatives. Russ. J. Gen. Chem. 2021, 91, 939–946. [Google Scholar] [CrossRef]
- Breitmaier, E. Structure Elucidation by NMR in Organic Chemistry: A Practical Guide, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2002. [Google Scholar]
- Kleinpeter, E. Quantification and Visualization of the Anisotropy Effect in NMR Spectroscopy by Through-Space NMR Shieldings. Annu. Rep. NMR Spectrosc. 2014, 82, 115–166. [Google Scholar]
- Nguyen, T.K.P.; Nguyen, K.P.P.; Kamounah, F.S.; Zhang, W.; Hansen, P.E. NMR of a series of novel hydroxyflavothiones. Magn. Reson. Chem. 2009, 47, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
| Atom | δ (13C), ppm | DEPT | δ (1H), ppm | Multiplicity (J, Hz) | 1H-1H COSY b | HMBC b |
|---|---|---|---|---|---|---|
| 3′ (NH) | 10.64 | s | 1′ | 2′, 5′, 4′/9 | ||
| 2′ (C=S) | 183.60 | C | ||||
| 1′ (NH) | 12.39 | s | 3′ | 2′, 5′, 4′/9 | ||
| 5′ (C=O) | 174.80 | C | ||||
| 4′/9 | 74.84 | C | ||||
| 1/8 | 123.76 | CH | 7.42 c | m | 2 | 2,3,4a,4′/9, 5′ e |
| 1a/8a | 141.40 | C | ||||
| 2/7 | 128.64 | CH | 7.39 c | m | 1, 3 | 1, 1a, 3, 4, 4′/9 |
| 3/6 | 130.27 | CH | 7.52 | m | 2, 4 | 1, 2, 4, 4a, 4′/9 e |
| 4/5 | 121.07 | CH | 7.93 | d (7.6) | 3 | 1, 2, 3, 4′/9 d, 1a |
| 4a/5a | 140.79 | C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoitsov, D.; Marinov, M.; Penchev, P.; Marinova, P.; Stoyanov, N. 5′-Oxospiro-(fluorene-9,4′-imidazolidine)-2′-thione. Molbank 2024, 2024, M1864. https://doi.org/10.3390/M1864
Stoitsov D, Marinov M, Penchev P, Marinova P, Stoyanov N. 5′-Oxospiro-(fluorene-9,4′-imidazolidine)-2′-thione. Molbank. 2024; 2024(3):M1864. https://doi.org/10.3390/M1864
Chicago/Turabian StyleStoitsov, Dimitar, Marin Marinov, Plamen Penchev, Petya Marinova, and Neyko Stoyanov. 2024. "5′-Oxospiro-(fluorene-9,4′-imidazolidine)-2′-thione" Molbank 2024, no. 3: M1864. https://doi.org/10.3390/M1864
APA StyleStoitsov, D., Marinov, M., Penchev, P., Marinova, P., & Stoyanov, N. (2024). 5′-Oxospiro-(fluorene-9,4′-imidazolidine)-2′-thione. Molbank, 2024(3), M1864. https://doi.org/10.3390/M1864







