3-((2-(4-Chloro-5-ethoxy-2-nitrophenoxy)acetamido)methyl)phenyl-dimethylcarbamate
Abstract
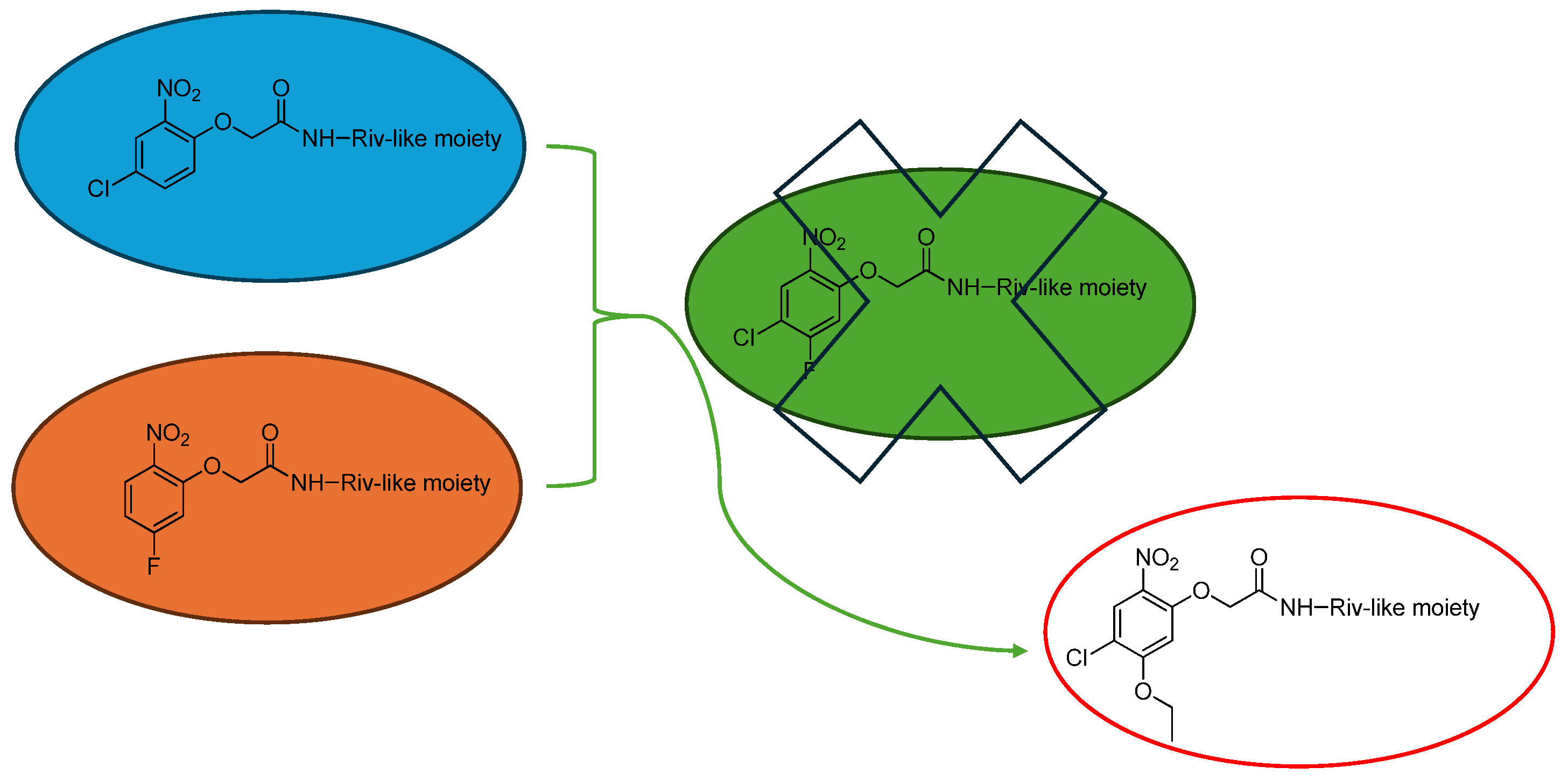
1. Introduction
2. Results and Discussion
3. Material and Methods
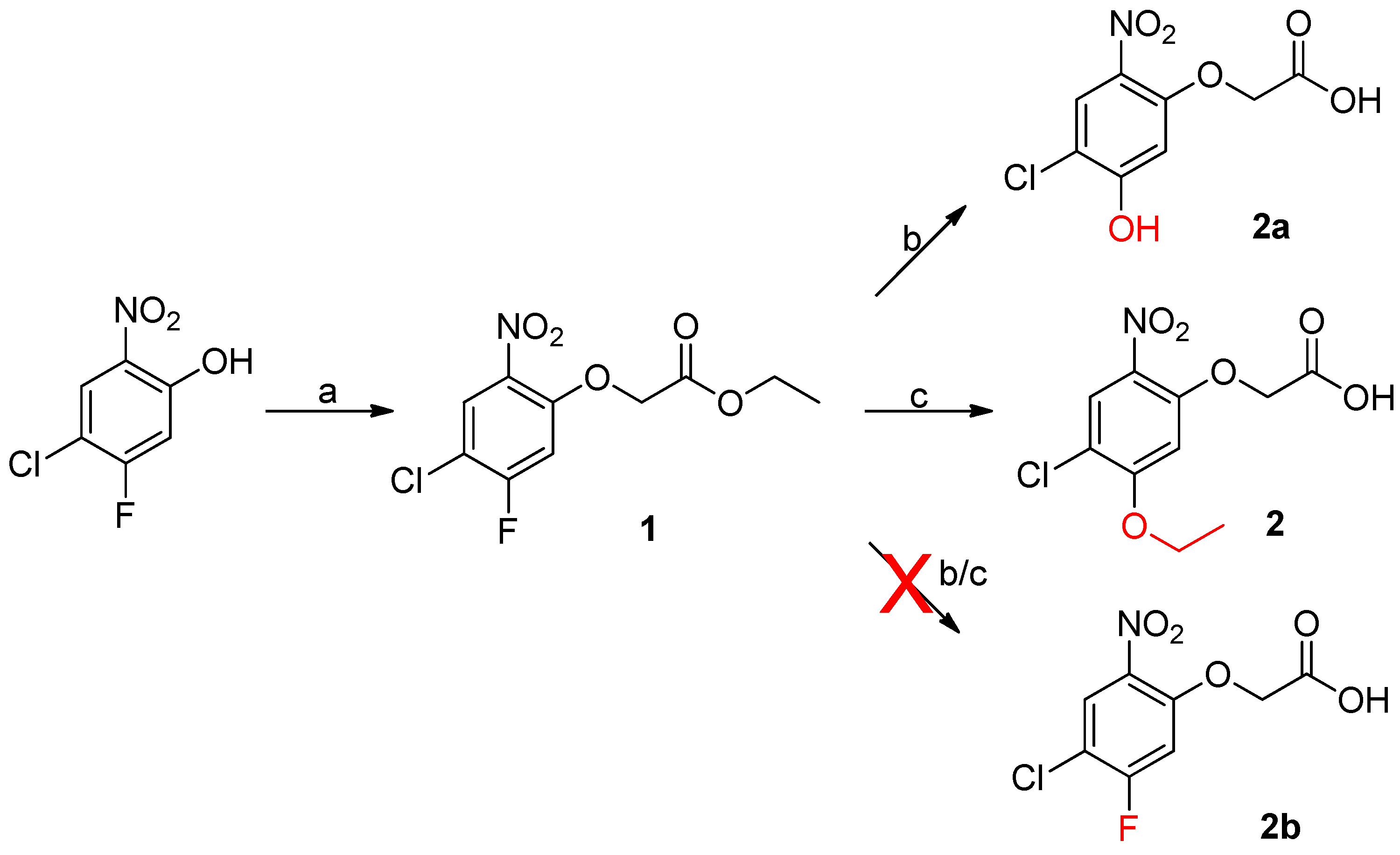
- Preparation of ethyl 2-(4-chloro-5-fluoro-2-nitrophenoxy)acetate (1)
- Preparation of 2-(4-chloro-5-ethoxy-2-nitrophenoxy)acetic acid (2)
- Preparation of 2-(4-chloro-5-hydroxy-2-nitrophenoxy)acetic acid (2a)
- Preparation of 3-cyanophenyl dimethylcarbamate (3)
- Preparation of 3-(aminomethyl)phenyl dimethyl carbamate (4)
- Preparation of 3-((2-(4-chloro-5-ethoxy-2-nitrophenoxy)acetamido)methyl)phenyl-dimethylcarbamate (5)
Supplementary Materials
Author Contributions
Funding

Acknowledgments
Conflicts of Interest
References
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Zetterberg, H. Biomarkers for Alzheimer’s Disease: Current Status and Prospects for the Future. J. Intern. Med. 2018, 284, 643–663. [Google Scholar] [CrossRef] [PubMed]
- Leuci, R.; Simic, S.; Carrieri, A.; Chaves, S.; La Spada, G.; Brunetti, L.; Tortorella, P.; Loiodice, F.; Laghezza, A.; Catto, M.; et al. Rivastigmine Structure-Based Hybrids as Potential Multi-Target Anti-Alzheimer’s Drug Candidates. Submitted.
- Brunetti, L.; Leuci, R.; Carrieri, A.; Catto, M.; Occhineri, S.; Vinci, G.; Gambacorta, L.; Baltrukevich, H.; Chaves, S.; Laghezza, A.; et al. Structure-Based Design of Novel Donepezil-like Hybrids for a Multi-Target Approach to the Therapy of Alzheimer’s Disease. Eur. J. Med. Chem. 2022, 237, 114358. [Google Scholar] [CrossRef] [PubMed]
- Sample, H.C.; Senge, M.O. Nucleophilic Aromatic Substitution (SNAr) and Related Reactions of Porphyrinoids: Mechanistic and Regiochemical Aspects. Eur. J. Org. Chem. 2021, 2021, 7–42. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, S.; Smith, A.J.; Pang, J.H.; Poole, D.L.; Tuttle, T.; Chiba, S.; Murphy, J.A. Concerted Nucleophilic Aromatic Substitution Reactions. Angew. Chem. Int. Ed. 2019, 58, 16368–16388. [Google Scholar] [CrossRef] [PubMed]
- Al-Amiery, A.A.; Al-Temimi, A.A.; Sulaiman, G.M.; Aday, H.A.; Kadhum, A.A.H.; Mohamad, A.B. Synthesis, Antimicrobial and Antioxidant Activities of 5-((2-Oxo-2H-Chromen-7-Yloxy)Methyl)-1,3,4-Thiadiazol-2(3H)-One Derived from Umbelliferone. Chem. Nat. Compd. 2013, 48, 950–954. [Google Scholar] [CrossRef]
- Chen, Z.; Digiacomo, M.; Tu, Y.; Gu, Q.; Wang, S.; Yang, X.; Chu, J.; Chen, Q.; Han, Y.; Chen, J.; et al. Discovery of Novel Rivastigmine-Hydroxycinnamic Acid Hybrids as Multi-Targeted Agents for Alzheimer’s Disease. Eur. J. Med. Chem. 2017, 125, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Hiremathad, A.; Chand, K.; Esteves, A.R.; Cardoso, S.M.; Ramsay, R.R.; Chaves, S.; Keri, R.S.; Santos, M.A. Tacrine-Allyl/Propargylcysteine–Benzothiazole Trihybrids as Potential Anti-Alzheimer’s Drug Candidates. RSC Adv. 2016, 6, 53519–53532. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leuci, R.; Dininno, D.; Paparella, M.; Piemontese, L. 3-((2-(4-Chloro-5-ethoxy-2-nitrophenoxy)acetamido)methyl)phenyl-dimethylcarbamate. Molbank 2024, 2024, M1863. https://doi.org/10.3390/M1863
Leuci R, Dininno D, Paparella M, Piemontese L. 3-((2-(4-Chloro-5-ethoxy-2-nitrophenoxy)acetamido)methyl)phenyl-dimethylcarbamate. Molbank. 2024; 2024(3):M1863. https://doi.org/10.3390/M1863
Chicago/Turabian StyleLeuci, Rosalba, Daniela Dininno, Marco Paparella, and Luca Piemontese. 2024. "3-((2-(4-Chloro-5-ethoxy-2-nitrophenoxy)acetamido)methyl)phenyl-dimethylcarbamate" Molbank 2024, no. 3: M1863. https://doi.org/10.3390/M1863
APA StyleLeuci, R., Dininno, D., Paparella, M., & Piemontese, L. (2024). 3-((2-(4-Chloro-5-ethoxy-2-nitrophenoxy)acetamido)methyl)phenyl-dimethylcarbamate. Molbank, 2024(3), M1863. https://doi.org/10.3390/M1863






