N-(2-(Benzylamino)ethyl)-4-(naphthalene-1-sulfonamido)benzamide
Abstract
1. Introduction
2. Results and Discussion
3. Material and Methods
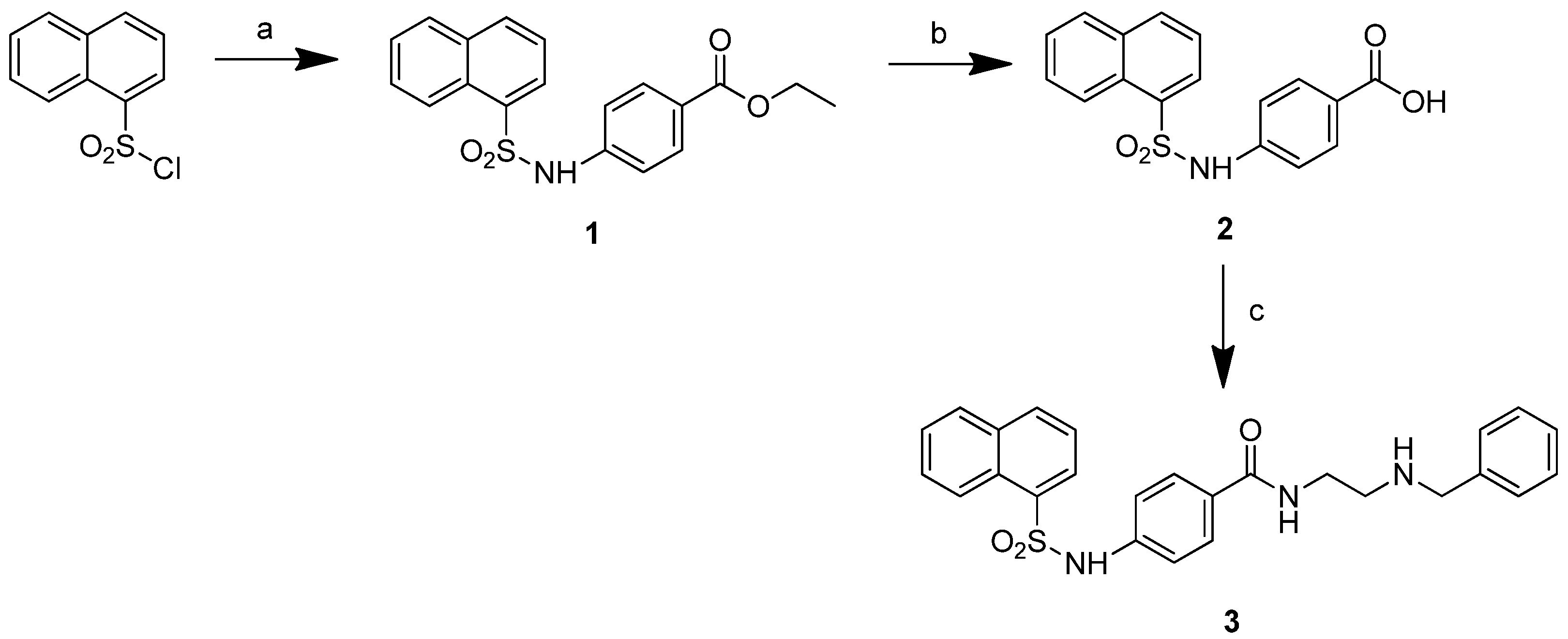
3.1. Preparation of Ethyl 4-(naphthalene-1-sulfonamido)benzoate (1)
3.2. Preparation of 4-(naphthalene-1-sulfonamido)benzoic Acid (2)
3.3. Preparation of N-(2-(Benzylamino)ethyl)-4-(naphthalene-1-sulfonamido)benzamide (3)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leuci, R.; Brunetti, L.; Laghezza, A.; Piemontese, L.; Carrieri, A.; Pisani, L.; Tortorella, P.; Catto, M.; Loiodice, F. A New Series of Aryloxyacetic Acids Endowed with Multi-Target Activity towards Peroxisome Proliferator-Activated Receptors (PPARs), Fatty Acid Amide Hydrolase (FAAH), and Acetylcholinesterase (AChE). Molecules 2022, 27, 958. [Google Scholar] [CrossRef] [PubMed]
- van Marrewijk, L.M.; Polyak, S.W.; Hijnen, M.; Kuruvilla, D.; Chang, M.R.; Shin, Y.; Kamenecka, T.M.; Griffin, P.R.; Bruning, J.B. SR2067 Reveals a Unique Kinetic and Structural Signature for PPARγ Partial Agonism. ACS Chem. Biol. 2016, 11, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Heydorn, W.E. Donepezil (E2020): A new acetylcholinesterase inhibitor. Review of its pharmacology, pharmacokinetics, and utility in the treatment of Alzheimer’s disease. Expert Opin. Investig. Drugs 1997, 6, 1527–1535. [Google Scholar] [CrossRef]
- Skulnick, H.I.; Johnson, P.D.; Aristoff, P.A.; Morris, J.K.; Lovasz, K.D.; Howe, W.J.; Watenpaugh, K.D.; Janakiraman, M.N.; Anderson, D.J.; Reischer, R.J.; et al. Structure-Based Design of Nonpeptidic HIV Protease Inhibitors: The Sulfonamide-Substituted Cyclooctylpyranones. J. Med. Chem. 1997, 40, 1149–1164. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, S.; Botta, L.; Semeraro, T.; Mugnaini, C.; Ligresti, A.; Palazzo, E.; Maione, S.; Di Marzo, V.; Corelli, F. Investigations on the 4-Quinolone-3-Carboxylic Acid Motif. 2. Synthesis and Structure-Activity Relationship of Potent and Selective Cannabinoid-2 Receptor Agonists Endowed with Analgesic Activity in Vivo. J. Med. Chem. 2008, 51, 5075–5084. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leuci, R.; Loiodice, F.; Piemontese, L. N-(2-(Benzylamino)ethyl)-4-(naphthalene-1-sulfonamido)benzamide. Molbank 2024, 2024, M1856. https://doi.org/10.3390/M1856
Leuci R, Loiodice F, Piemontese L. N-(2-(Benzylamino)ethyl)-4-(naphthalene-1-sulfonamido)benzamide. Molbank. 2024; 2024(3):M1856. https://doi.org/10.3390/M1856
Chicago/Turabian StyleLeuci, Rosalba, Fulvio Loiodice, and Luca Piemontese. 2024. "N-(2-(Benzylamino)ethyl)-4-(naphthalene-1-sulfonamido)benzamide" Molbank 2024, no. 3: M1856. https://doi.org/10.3390/M1856
APA StyleLeuci, R., Loiodice, F., & Piemontese, L. (2024). N-(2-(Benzylamino)ethyl)-4-(naphthalene-1-sulfonamido)benzamide. Molbank, 2024(3), M1856. https://doi.org/10.3390/M1856





