1-(2-(3,5-Di-tert-butyl-4-hydroxyphenyl)-2-oxoethyl) Quinolin-1-ium Bromide
Abstract
1. Introduction
2. Results and Discussion
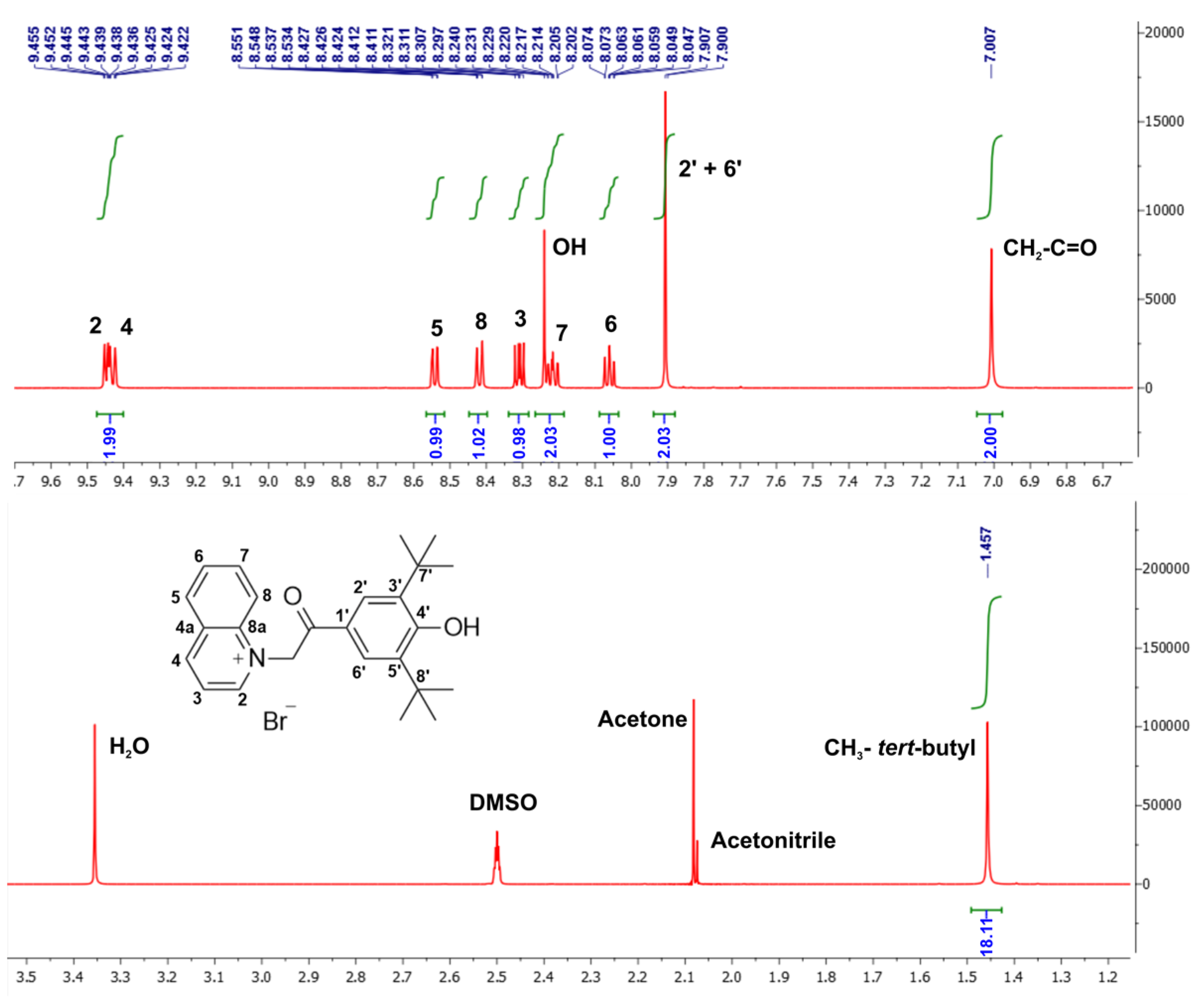
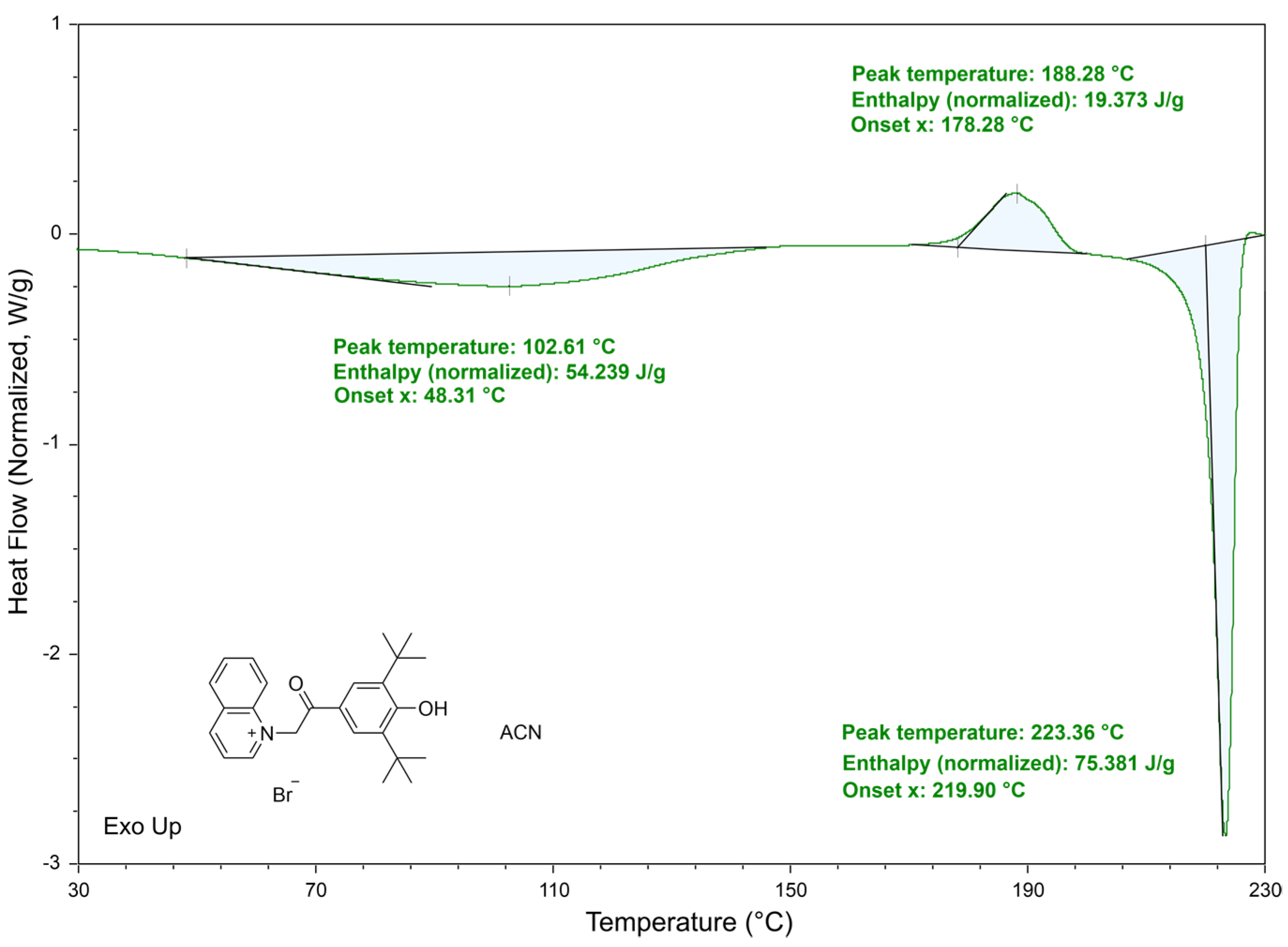
2.1. Synthesis and Characterisation
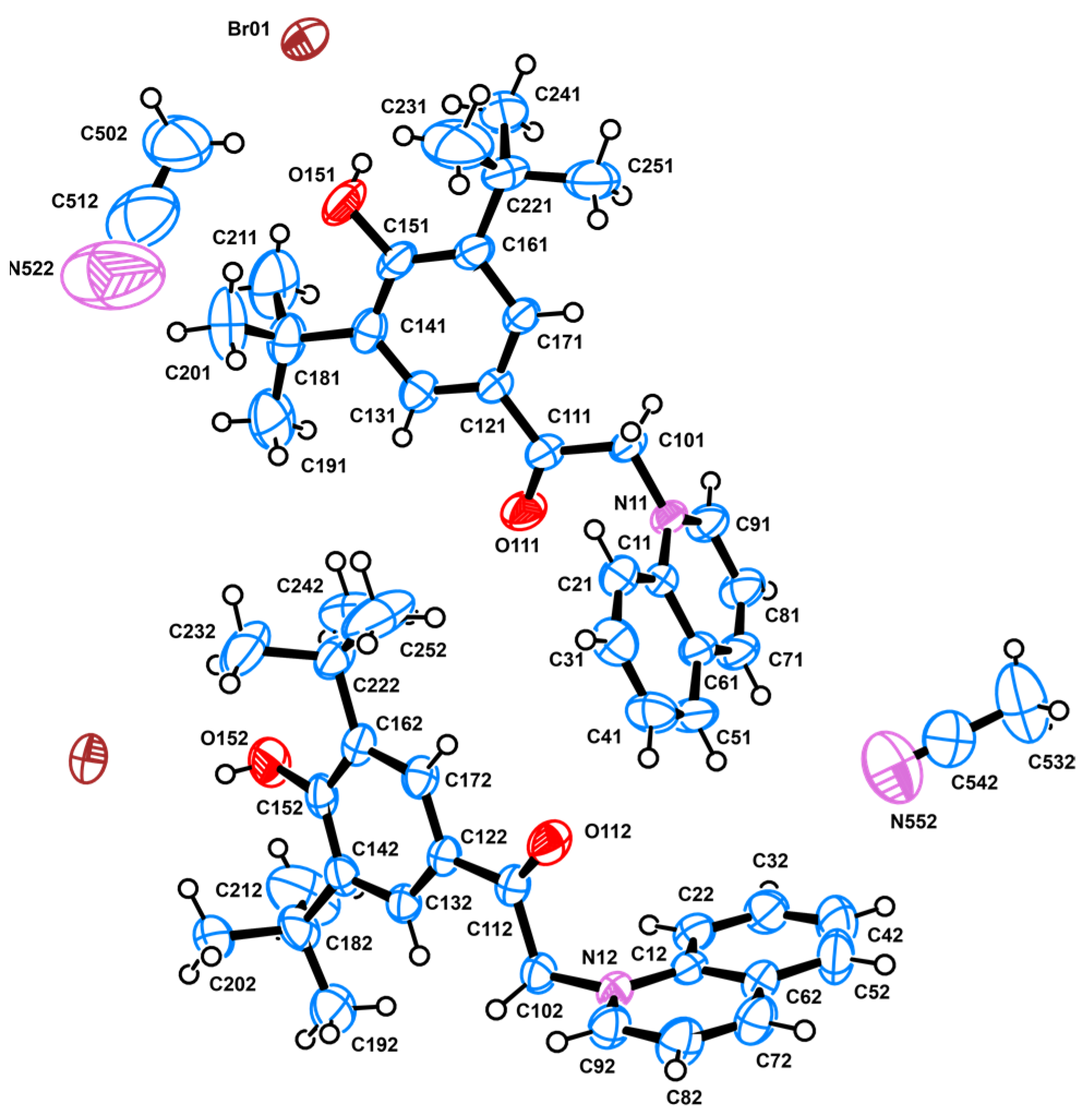
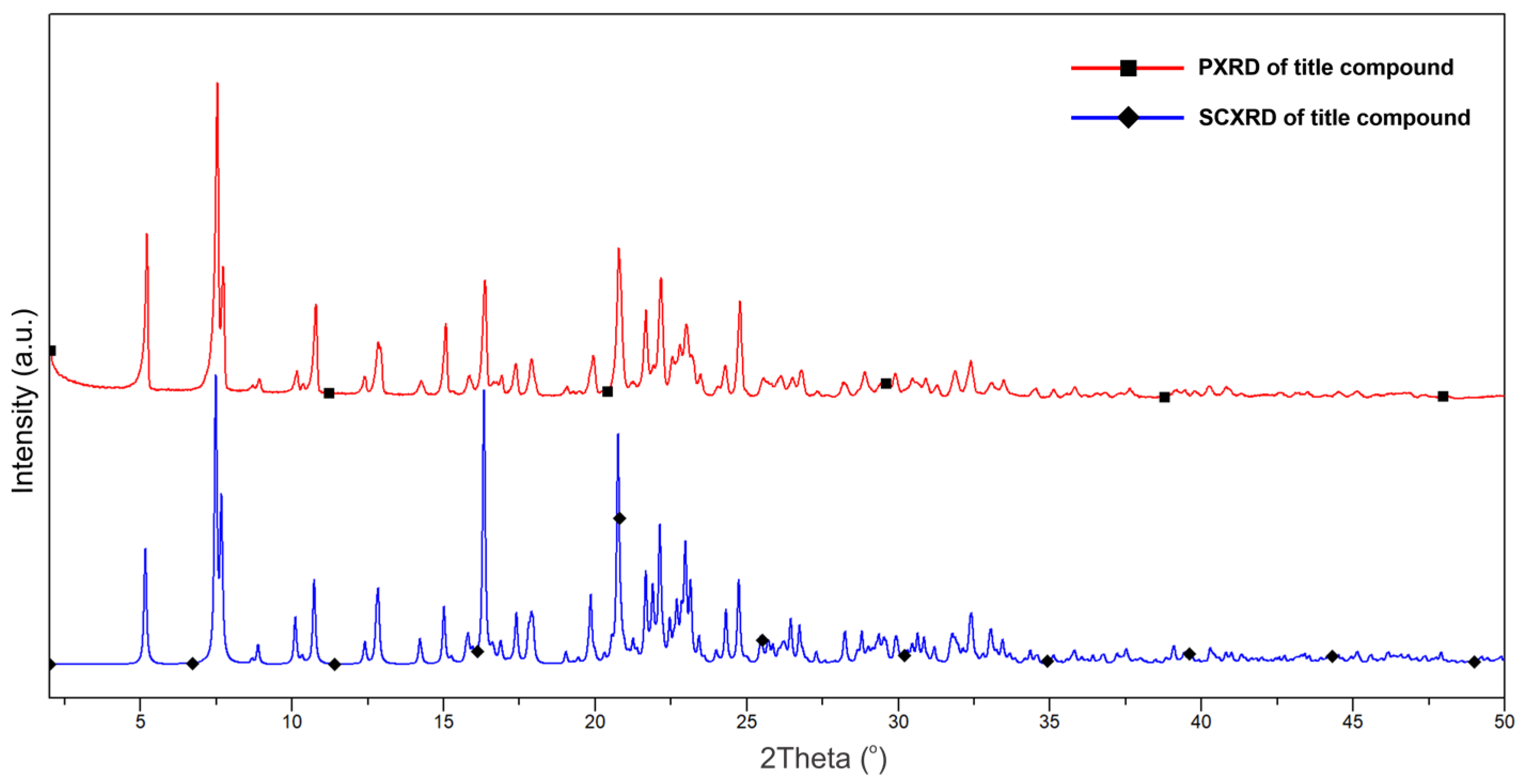
2.2. Crystallography
3. Materials and Methods
3.1. General
3.2. Synthesis of 1-(2-(3,5-Di-tert-butyl-4-hydroxyphenyl)-2-oxoethyl) Quinolin-1-ium Bromide
3.3. Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abboud, J.-L.M.; Notario, R.; Bertrán, J.; Solà, M. One century of physical organic chemistry: The Menshutkin reaction. In Progress in Physical Organic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 1993; Volume 19. [Google Scholar]
- Arnett, E.M.; Reich, R. Electronic effects on the Menshutkin reaction. A complete kinetic and thermodynamic dissection of alkyl transfer to 3-and 4-substituted pyridines. J. Am. Chem. Soc. 1980, 102, 5892–5902. [Google Scholar] [CrossRef]
- Sola, M.; Lledos, A.; Duran, M.; Bertran, J.; Abboud, J.L.M. Analysis of solvent effects on the Menshutkin reaction. J. Am. Chem. Soc. 1991, 113, 2873–2879. [Google Scholar] [CrossRef]
- Castejon, H.; Wiberg, K.B. Solvent effects on methyl transfer reactions. 1. The Menshutkin reaction. J. Am. Chem. Soc. 1999, 121, 2139–2146. [Google Scholar] [CrossRef]
- Lewinska, G.; Sanetra, J.; Marszalek, K.W. Application of quinoline derivatives in third-generation photovoltaics. J. Mater. Sci. Mater. Electron. 2021, 32, 18451–18465. [Google Scholar] [CrossRef]
- Sivaraman, G.; Iniya, M.; Anand, T.; Kotla, N.G.; Sunnapu, O.; Singaravadivel, S.; Gulyani, A.; Chellappa, D. Chemically diverse small molecule fluorescent chemosensors for copper ion. Coord. Chem. Rev. 2018, 357, 50–104. [Google Scholar] [CrossRef]
- Matada, B.S.; Pattanashettar, R.; Yernale, N.G. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem. 2021, 32, 115973. [Google Scholar] [CrossRef]
- Yadav, P.; Shah, K. Quinolines, a perpetual, multipurpose scaffold in medicinal chemistry. Bioorg. Chem. 2021, 109, 104639. [Google Scholar] [CrossRef] [PubMed]
- El Shehry, M.F.; Ghorab, M.M.; Abbas, S.Y.; Fayed, E.A.; Shedid, S.A.; Ammar, Y.A. Quinoline derivatives bearing pyrazole moiety: Synthesis and biological evaluation as possible antibacterial and antifungal agents. Eur. J. Med. Chem. 2018, 143, 1463–1473. [Google Scholar] [CrossRef]
- Yeo, S.-J.; Liu, D.-X.; Kim, H.S.; Park, H. Anti-malarial effect of novel chloroquine derivatives as agents for the treatment of malaria. Malar. J. 2017, 16, 80. [Google Scholar] [CrossRef]
- Quimque, M.T.J.; Go, A.D.; Lim, J.A.K.; Vidar, W.S.; Macabeo, A.P.G. Mycobacterium tuberculosis inhibitors based on arylated quinoline carboxylic acid backbones with anti-mtb gyrase activity. Int. J. Mol. Sci. 2023, 24, 11632. [Google Scholar] [CrossRef]
- Abdelrahman, M.A.; Almahli, H.; Al-Warhi, T.; Majrashi, T.A.; Abdel-Aziz, M.M.; Eldehna, W.M.; Said, M.A. Development of novel isatin-tethered quinolines as anti-tubercular agents against multi and extensively drug-resistant mycobacterium tuberculosis. Molecules 2022, 27, 8807. [Google Scholar] [CrossRef] [PubMed]
- Mantu, D.; Antoci, V.; Moldoveanu, C.; Zbancioc, G.; Mangalagiu, I.I. Hybrid imidazole (benzimidazole)/pyridine (quinoline) derivatives and evaluation of their anticancer and antimycobacterial activity. J. Enzym. Inhib. Med. Chem. 2016, 31, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Bingul, M.; Tan, O.; Gardner, C.R.; Sutton, S.K.; Arndt, G.M.; Marshall, G.M.; Cheung, B.B.; Kumar, N.; Black, D.S. Synthesis, characterization and anti-cancer activity of hydrazide derivatives incorporating a quinoline moiety. Molecules 2016, 21, 916. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.I.; Farrag, A.M.; Abbas, S.Y.; El Shehry, M.F.; Ragab, A.; Fayed, E.A.; Ammar, Y.A. Novel structural hybrids of quinoline and thiazole moieties: Synthesis and evaluation of antibacterial and antifungal activities with molecular modeling studies. Bioorg. Chem. 2021, 110, 104803. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.-G.; Li, Z.-W.; Hu, X.-X.; Si, S.-Y.; You, X.-F.; Tang, S.; Wang, Y.-X.; Song, D.-Q. Synthesis and biological evaluation of quinoline derivatives as a novel class of broad-spectrum antibacterial agents. Molecules 2019, 24, 548. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Dash, S.P.; Mohanty, M.; Sanna, D.; Sciortino, G.; Ugone, V.; Garribba, E.; Reuter, H.; Kaminsky, W.; Dinda, R. Chemistry of mixed-ligand oxidovanadium (iv) complexes of aroylhydrazones incorporating quinoline derivatives: Study of solution behavior, theoretical evaluation and protein/DNA interaction. J. Inorg. Biochem. 2019, 199, 110786. [Google Scholar] [CrossRef] [PubMed]
- El-Sonbati, A.Z.; Diab, M.A.; Morgan, S.M.; Abou-Dobara, M.I.; El-Ghettany, A.A. Synthesis, characterization, theoretical and molecular docking studies of mixed-ligand complexes of cu(ii), ni(ii), co(ii), mn(ii), cr(iii), uo2(ii) and cd(ii). J. Mol. Struct. 2020, 1200, 127065. [Google Scholar] [CrossRef]
- Elbatrawy, A.A.; Hyeon, S.J.; Yue, N.; Osman, E.E.A.; Choi, S.H.; Lim, S.; Kim, Y.K.; Ryu, H.; Cui, M.; Nam, G. “Turn-on” quinoline-based fluorescent probe for selective imaging of tau aggregates in alzheimer’s disease: Rational design, synthesis, and molecular docking. ACS Sens. 2021, 6, 2281–2289. [Google Scholar] [CrossRef]
- Pradhan, A.B.; Mandal, S.K.; Banerjee, S.; Mukherjee, A.; Das, S.; Bukhsh, A.R.K.; Saha, A. A highly selective fluorescent sensor for zinc ion based on quinoline platform with potential applications for cell imaging studies. Polyhedron 2015, 94, 75–82. [Google Scholar] [CrossRef]
- Yao, Y.; Ding, P.; Yan, C.; Tao, Y.; Peng, B.; Liu, W.; Wang, J.; Stuart, M.A.C.; Guo, Z. Fluorescent probes based on aiegen-mediated polyelectrolyte assemblies for manipulating intramolecular motion and magnetic relaxivity. Angew. Chem. Int. Ed. 2023, 62, e202218983. [Google Scholar] [CrossRef]
- Tselekidou, D.; Panagiotidis, L.; Papadopoulos, K.; Kyriazopoulos, V.; Gioti, M. A comparative study of ir (dmpq) 2 (acac) doped cbp, mcp, tapc and tcta for phosphorescent oleds. Photonics 2022, 9, 800. [Google Scholar] [CrossRef]
- Ali, B.; Khalid, M.; Asim, S.; Khan, M.U.; Iqbal, Z.; Hussain, A.; Hussain, R.; Ahmed, S.; Ali, A.; Hussain, A. Key electronic, linear and nonlinear optical properties of designed disubstituted quinoline with carbazole compounds. Molecules 2021, 26, 2760. [Google Scholar] [CrossRef] [PubMed]
- Daoud, D.; Hamani, H.; Douadi, T. Novel heterocyclic quinoline derivatives as green environmental corrosion inhibitors for carbon steel in hcl solution: Experimental and theoretical investigation. J. Adhes. Sci. Technol. 2021, 35, 2319–2345. [Google Scholar] [CrossRef]
- Abdelshafi, N.; Ibrahim, M.A.; Badran, A.-S.; Halim, S.A. Experimental and theoretical evaluation of a newly synthesized quinoline derivative as corrosion inhibitor for iron in 1.0 m hydrochloric acid solution. J. Mol. Struct. 2022, 1250, 131750. [Google Scholar] [CrossRef]
- Nikitin, E.; Mironova, E.; Shpakovsky, D.; Gracheva, Y.; Koshelev, D.; Utochnikova, V.; Lyssenko, K.; Oprunenko, Y.; Yakovlev, D.; Litvinov, R. Cytotoxic and luminescent properties of novel organotin complexes with chelating antioxidant ligand. Molecules 2022, 27, 8359. [Google Scholar] [CrossRef] [PubMed]
- Osipova, V.; Gracheva, Y.; Polovinkina, M.; Burmistrova, D.; Berberova, N. Antioxidant activity and cytotoxicity of aromatic oligosulfides. Molecules 2022, 27, 3961. [Google Scholar] [CrossRef] [PubMed]
- Koshelev, V.N.; Primerova, O.V.; Vorobyev, S.V.; Ivanova, L.V. Synthesis, redox properties and antibacterial activity of hindered phenols linked to heterocycles. Molecules 2020, 25, 2370. [Google Scholar] [CrossRef]
- Ariffin, A.; Rahman, N.A.; Yehye, W.A.; Alhadi, A.A.; Kadir, F.A. Pass-assisted design, synthesis and antioxidant evaluation of new butylated hydroxytoluene derivatives. Eur. J. Med. Chem. 2014, 87, 564–577. [Google Scholar] [CrossRef]
- Kong, C.; Yehye, W.A.; Rahman, N.A.; Tan, M.-W.; Nathan, S. Discovery of potential anti-infectives against staphylococcus aureus using a caenorhabditis elegans infection model. BMC Complement Altern. Med. 2014, 14, 4. [Google Scholar] [CrossRef]
- Shakir, R.M.; Ariffin, A.; Abdulla, M.A. Synthesis of new 2, 5-di-substituted 1, 3, 4-oxadiazoles bearing 2, 6-di-tert-butylphenol moieties and evaluation of their antioxidant activity. Molecules 2014, 19, 3436–3449. [Google Scholar] [CrossRef]
- Milaeva, E.; Filimonova, S.; Meleshonkova, N.; Dubova, L.; Shevtsova, E.; Bachurin, S.; Zefirov, N. Antioxidative activity of ferrocenes bearing 2, 6-di-tert-butylphenol moieties. Bioinorg. Chem. Appl. 2010, 2010, 165482. [Google Scholar] [CrossRef] [PubMed]
- Gibadullina, E.; Thu, N.T.; Bukharov, S.; Burilov, A. Synthesis of sterically hindered phenols based on 7-amino-2, 4-dimethylqunoline and 5, 7-dimethyl-1, 8-naphthyridin-2-amine. Russ. J. Gen. Chem. 2017, 87, 1305–1309. [Google Scholar] [CrossRef]
- Mikhalev, O.; Shpakovsky, D.; Gracheva, Y.A.; Antonenko, T.; Albov, D.; Aslanov, L.; Milaeva, E. Synthesis and study of new phenolic antioxidants with nitroaromatic and heterocyclic substituents. Russ. Chem. Bull. 2018, 67, 712–720. [Google Scholar] [CrossRef]
- Dudognon, E.; Correia, N.T.; Danède, F.; Descamps, M. Solid-solid transformation in racemic ibuprofen. Pharm. Res. 2013, 30, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Macedi, E.; Paoli, P.; Bernazzani, L.; Carignani, E.; Borsacchi, S.; Geppi, M. Solid–solid transition between hydrated racemic compound and anhydrous conglomerate in na-ibuprofen: A combined X-ray diffraction, solid-state NMR, calorimetric, and computational study. Cryst. Growth Des 2014, 14, 2441–2452. [Google Scholar] [CrossRef]
- Farrugia, L.J. Wingx and ortep for windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Bruker, A. Saint and Sadabs; Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Bruker, A. Apex 4; Bruker AXS Inc.: Madison, WI, USA, 2022. [Google Scholar]
- Sheldrick, G.M. Shelxt–integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with shelxl. Acta Crystallogr. Sect. C 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. Olex2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusew, R.; Iliev, K.; Kurteva, V.; Shivachev, B. 1-(2-(3,5-Di-tert-butyl-4-hydroxyphenyl)-2-oxoethyl) Quinolin-1-ium Bromide. Molbank 2024, 2024, M1763. https://doi.org/10.3390/M1763
Rusew R, Iliev K, Kurteva V, Shivachev B. 1-(2-(3,5-Di-tert-butyl-4-hydroxyphenyl)-2-oxoethyl) Quinolin-1-ium Bromide. Molbank. 2024; 2024(1):M1763. https://doi.org/10.3390/M1763
Chicago/Turabian StyleRusew, Rusi, Kostadin Iliev, Vanya Kurteva, and Boris Shivachev. 2024. "1-(2-(3,5-Di-tert-butyl-4-hydroxyphenyl)-2-oxoethyl) Quinolin-1-ium Bromide" Molbank 2024, no. 1: M1763. https://doi.org/10.3390/M1763
APA StyleRusew, R., Iliev, K., Kurteva, V., & Shivachev, B. (2024). 1-(2-(3,5-Di-tert-butyl-4-hydroxyphenyl)-2-oxoethyl) Quinolin-1-ium Bromide. Molbank, 2024(1), M1763. https://doi.org/10.3390/M1763






