(2-[4-Methylpyrazol-1-yl]phenyl)platinum(II) (1,3-bis[1-methyl-1H-pyrazol-4-yl]propane-1,3-dione)
Abstract
1. Introduction
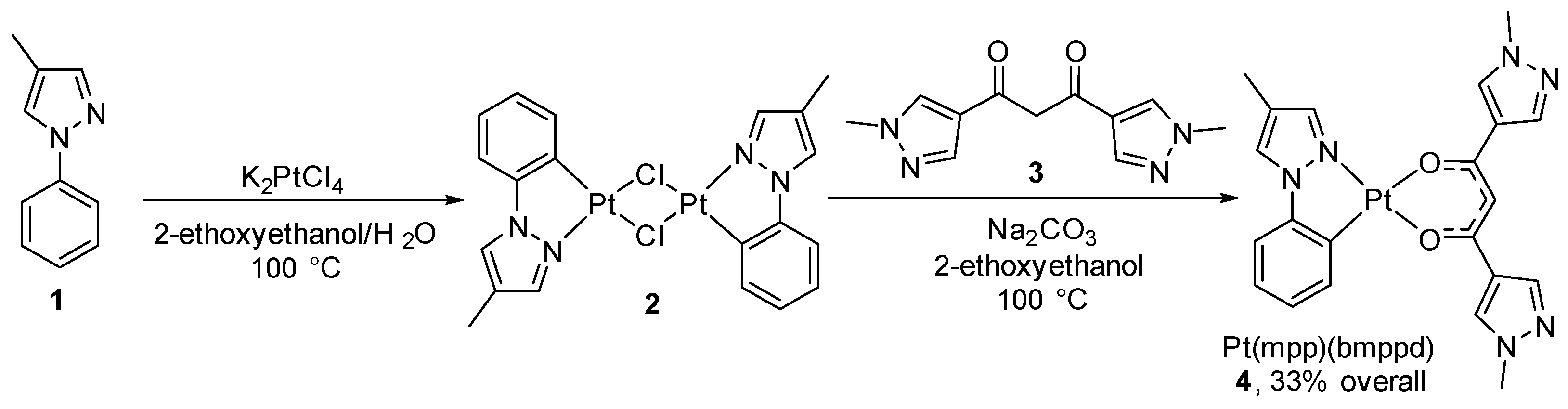
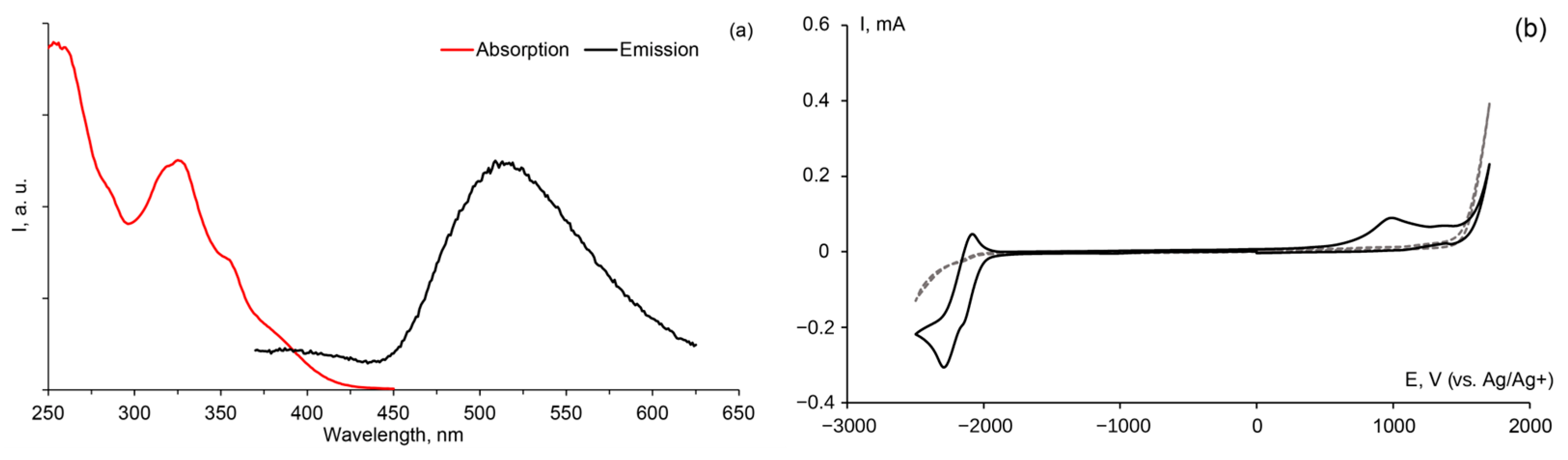
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. (2-[4-Methylpyrazol-1-yl]phenyl)platinum(II) (1,3-bis[1-methyl-1H-pyrazol-4-yl]propane-1,3-dione) (Pt(mpp)(bmppd)) (4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chi, Y.; Chou, P.-T. Transition-metal phosphors with cyclometalating ligands: Fundamentals and applications. Chem. Soc. Rev. 2010, 39, 638–655. [Google Scholar] [CrossRef] [PubMed]
- Jayabharathi, J.; Thanikachalam, V.; Thilagavathy, S. Phosphorescent organic light-emitting devices: Iridium based emitter materials—An overview. Coord. Chem. Rev. 2023, 483, 215100. [Google Scholar] [CrossRef]
- Jain, V.K. Cyclometalated group-16 compounds of palladium and platinum: Challenges and opportunities. Coord. Chem. Rev. 2021, 427, 213546. [Google Scholar] [CrossRef]
- Cebrián, C.; Mauro, M. Recent advances in phosphorescent platinum complexes for organic light-emitting diodes. Beilstein J. Org. Chem. 2018, 14, 1459–1481. [Google Scholar] [CrossRef] [PubMed]
- Shigehiro, T.; Chen, Q.; Yagi, S.; Maeda, T.; Nakazumi, H.; Sakurai, Y. Substituent effect on photo- and electroluminescence properties of heteroleptic cyclometalated platinum(II) complexes based on a 2-(dibenzo[b,d]furan-4-yl)pyridine ligand. Dyes Pigment. 2016, 124, 165–173. [Google Scholar] [CrossRef]
- Haque, A.; El Moll, H.; Alenezi, K.M.; Khan, M.S.; Wong, W.-Y. Functional Materials Based on Cyclometalated Platinum(II) β-Diketonate Complexes: A Review of Structure–Property Relationships and Applications. Materials 2021, 14, 4236. [Google Scholar] [CrossRef] [PubMed]
- Taidakov, I.; Ambrozevich, S.; Saifutyarov, R.; Lyssenko, K.; Avetisov, R.; Mozhevitina, E.; Khomyakov, A.; Khrizanforov, M.; Budnikova, Y.; Avetissov, I. New Pt(II) complex with extra pure green emission for OLED application: Synthesis, crystal structure and spectral properties. J. Organomet. Chem. 2018, 867, 253–260. [Google Scholar] [CrossRef]
- Pavlik, J.W.; Connors, R.E.; Burns, D.S.; Kurzweil, E.M. Phototransposition chemistry of 1-phenylpyrazole. Experimental and computational studies. J. Am. Chem. Soc. 1993, 115, 7645–7652. [Google Scholar] [CrossRef]
- Taydakov, I.V.; Krasnoselsky, S.S. Modified method for the synthesis of isomeric N-substituted (1H-pyrazolyl)propane-1,3-diones. Chem. Heterocycl. Compd. 2011, 47, 695–699. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlasova, T.S.; Romanova, Y.E.; Taidakov, I.V.; Paveliev, S.A. (2-[4-Methylpyrazol-1-yl]phenyl)platinum(II) (1,3-bis[1-methyl-1H-pyrazol-4-yl]propane-1,3-dione). Molbank 2024, 2024, M1764. https://doi.org/10.3390/M1764
Vlasova TS, Romanova YE, Taidakov IV, Paveliev SA. (2-[4-Methylpyrazol-1-yl]phenyl)platinum(II) (1,3-bis[1-methyl-1H-pyrazol-4-yl]propane-1,3-dione). Molbank. 2024; 2024(1):M1764. https://doi.org/10.3390/M1764
Chicago/Turabian StyleVlasova, Tatiana S., Yulia E. Romanova, Ilya V. Taidakov, and Stanislav A. Paveliev. 2024. "(2-[4-Methylpyrazol-1-yl]phenyl)platinum(II) (1,3-bis[1-methyl-1H-pyrazol-4-yl]propane-1,3-dione)" Molbank 2024, no. 1: M1764. https://doi.org/10.3390/M1764
APA StyleVlasova, T. S., Romanova, Y. E., Taidakov, I. V., & Paveliev, S. A. (2024). (2-[4-Methylpyrazol-1-yl]phenyl)platinum(II) (1,3-bis[1-methyl-1H-pyrazol-4-yl]propane-1,3-dione). Molbank, 2024(1), M1764. https://doi.org/10.3390/M1764





