Abstract
The reaction of 2-bromo-1-(5-methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)ethan-1-one (1) with 4-amino-5-((2,4-dichlorophenoxy)methyl)-4H-1,2,4-triazole-3-thiol (2) in absolute ethanol in the presence of triethyl amine as catalyst gave 2-((4-amino-5-((2,4-dichlorophenoxy)methyl)-4H-1,2,4-triazol-3-yl)thio)-1-(5-methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)ethan-1-one (3) in 73% yield. The structure of the title heterocycle (3) was confirmed by X-ray single crystal diffraction and spectral analyses (NMR and IR).
1. Introduction
Heterocycles containing the 1,2,3-triazole fragment are active ingredients in a variety of drugs, including antibiotics (e.g., cefatrizine and tazobactam). 1,2,3-triazole derivatives exhibit a variety of promising biological properties, including antibacterial, antituberculosis, antiviral, and anticancer properties [1,2,3,4,5].
The diverse pharmacological activities of 1,2,4-triazoles as fungicides, antivirals, herbicides, and catalase inhibitors have led to deep interest in discovering new entities for their broader applications. There are many drugs based on 1,2,4-triazole in clinical use for the treatment of various diseases, such as antifungals (fluconazole, itraconazole, and voriconazole) and antivirals (rifavirin) [6,7,8,9].
Therefore, the current work reports the synthesis and crystal structure of a new heterocycle containing both 1,2,3-triazole and 1,2,4-triazole moieties using a simple procedure. Recently, the synthesis and structure elucidation of new heterocycles have been reported [10,11].
2. Results and Discussion
2.1. Synthesis
The heating under reflux conditions of 2-bromo-1-(5-methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)ethan-1-one (1) with 4-amino-5-((2,4-dichlorophenoxy)methyl)-4H-1,2,4-triazole-3-thiol (2) in absolute ethanol in the presence of triethyl amine as catalyst gave 2-((4-amino-5-((2,4-dichlorophenoxy)methyl)-4H-1,2,4-triazol-3-yl)thio)-1-(5-methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)ethan-1-one (3) in 73% yield (Scheme 1). Crystallization of the solid obtained from dimethylformamide (DMF) gave 3 in crystal form.
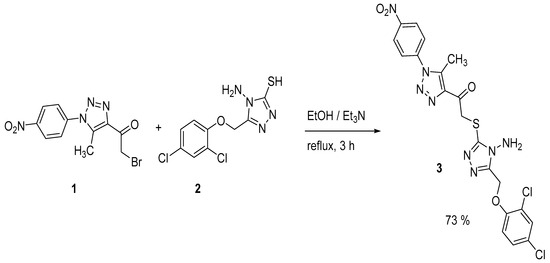
Scheme 1.
Synthesis of heterocycle 3.
2.2. NMR Spectroscopy
The 1H NMR spectrum of 3 showed both the methylene protons (CH2) as a singlet signal at 4.95 and 6.10 ppm and the presence of a singlet signal that appeared at 5.30 ppm due to the NH2 protons. We cannot perform the 13C NMR due to the poor solubility of product 3.
2.3. Crystal Structure Analysis
An ortep view of the geometry of the title structure can be seen in Figure 1. The structure typically comprises two formulae in the unit cell and a monoclinic crystal system with the P21/c space group. The molecule geometry is in good agreement with the reported standard bond distance [12].
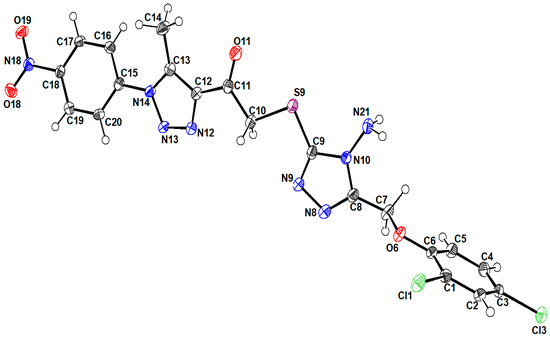
Figure 1.
A view of the structure of the title compound, showing the atom-labeling scheme. Displacement ellipsoids are drawn at the 50% probability level.
The constituents of the structure showed a planar appearance for each component separately. However, torsion angles between the consisting moieties give a non-planar appearance to the compound, such as C6/O6/C7/C8 (178.6°), N13/N14/C15/C20 (25.50°), and N9/C9/S9/C10 (5.76°). Also, the angle between the planes of the rings (C9 N9 N8 C8 N10) and (C5 C4 C3 C2 C1 C6) is 85°.
Figure 2 shows that the structure is stabilized by hydrogen bonds between the carbon atom and the nitrogen, oxygen, and chlorine atoms, as follows: C7—H7B---N9, C7—H7A---O11, C10—H10B---O11 and C20—H20---Cl3 together. Beautiful parallel networks with a wavy pattern could be seen in the crystal packing (Figure 3).
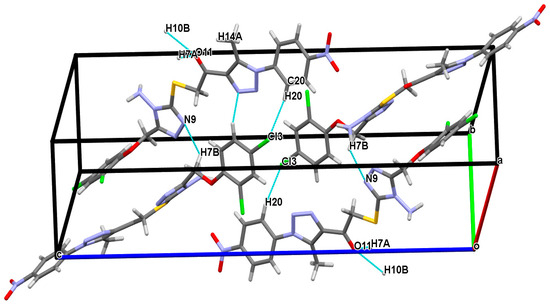
Figure 2.
A segment of the structure showing hydrogen bonding as blue dotted lines.
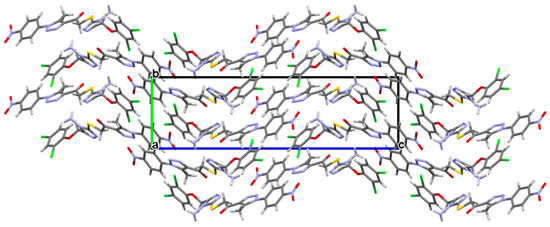
Figure 3.
A view of crystal packing of the title compound along a-axis.
3. Materials and Methods
3.1. General
Chemicals and solvents were obtained from Merck. The IR was recorded on Bruker Tensor 27 FTIR spectroscope. The NMR spectra (600 MHz) of 3 were performed on a Bruker NMR spectrometer. The chemical shift (δ) was reported in ppm. The NMR spectra were recorded in DMSO-d6. The literature procedures were used to prepare 1 [13] and 2 [14].
3.2. Synthesis of 3
A mixture of 1 (0.65 g, 2.0 mmol), 2 (0.58 g, 2.0 mmol), and triethyl amine (0.2 mL) in absolute ethanol (15 mL) was heated under reflux conditions for 3 h and left to cool to at room temperature. Then, the colorless solid produced was collected by filtration. The product was washed with EtOH, dried, and recrystallized from DMF to give 3 in 73% yield, mp 230–231 °C. IR: 3345 (NH2), 3171-2915 (CH), 1681 (C=O),1613-1596(C=N) cm−1. 1H NMR: 2.55 (s, 3H, Me), 4.95 (s, 2H, CH2), 5.30 (s, 2H, NH2), 6.10 (s, 2H, CH2), 7.40 (s, 2H, Ar), 7.69 (s, 1H, Ar), 8.04 (d, 2H, J= 9 MHz, Ar), 8.50 (d, J= 9 MHz, 2H, Ar). Anal. Calcd. for C20H16Cl2N8O4S (535.36): C, 44.87; H, 3.01; N, 20.93. Found: C, 44.93; H, 3.19; N, 20.99%.
3.3. X-ray Crystallography
A Bruker APEX-II CCD X-ray diffractometer (Mo X-ray tube) was used to collect data from a colorless plate crystal that had approximate dimensions of 0.286 × 0.274 × 0.046 mm3 [15]. The structure was solved and refined using SHELXT [16] and SHELXL [17]. The structure was analyzed and graphically demonstrated using the crystallographic computer programs PLATON [18], Mercury [19], and ORTEP-3 for Windows [20].
The full crystallographic details are Ided in the Supplementary Materials (CIF and Tables S1–S3). The data have been deposited in the Cambridge Crystallographic Data Centre (CSD) with the number CCDC 2288217.
4. Conclusions
A new heterocycle containing both 1,2,3-triazole and 1,2,4-triazole moieties has been synthesized with good yield using a simple procedure. The structure of the title heterocycle was established using X-ray single crystal diffraction and nuclear magnetic resonance spectroscopy.
Supplementary Materials
The following supporting information are available online, 1H NMR spectra, CIFs, and checkcif reports for heterocycle 3.
Author Contributions
Conceptualization: B.F.A.-W. and A.A.F.; methodology: J.C.F. and A.F.M.; X-ray crystal structure. investigation: B.F.A.-W., A.F.M., J.C.F. and A.A.F.; writing—original draft preparation: B.F.A.-W., A.F.M., J.C.F. and A.A.F.; writing—review and editing: B.F.A.-W., A.F.M., J.C.F. and A.A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article and the Supplementary Material.
Acknowledgments
We thank the National Research Centre and Cardiff University for their technical support.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
A sample of the title compound is available from the authors.
References
- Liang, T.; Sun, X.; Li, W.; Hou, G.; Gao, F. 1,2,3-Triazole-Containing Compounds as Anti–Lung Cancer Agents: Current Developments, Mechanisms of Action, and Structure–Activity Relationship. Front. Pharmacol. 2021, 12, 661173. [Google Scholar] [CrossRef] [PubMed]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Akhtar, M.J.; Gogoi, U.; Meenakshi, D.U.; Das, A. An Overview of 1,2,3-triazole-Containing Hybrids and Their Potential Anticholinesterase Activities. Pharmaceuticals 2023, 16, 179. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Khokra, S.L.; Yadav, A. Triazole analogues as potential pharmacological agents: A brief review. Futur. J. Pharm. Sci. 2021, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Kabi, A.K.; Sravani, S.; Gujjarappa, R.; Garg, A.; Vodnala, N.; Tyagi, U.; Kaldhi, D.; Singh, V.; Gupta, S.; Malakar, C.C. An Overview on Biological Activities of 1,2,3-Triazole Derivatives. In Nanostructured Biomaterials: Basic Structures and Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 401–423. [Google Scholar] [CrossRef]
- Abdelli, A.; Azzouni, S.; Plais, R.; Gaucher, A.; Efrit, M.L.; Prim, D. Recent advances in the chemistry of 1,2,4-triazoles: Synthesis, reactivity and biological activities. Tetrahedron Lett. 2021, 86, 153518. [Google Scholar] [CrossRef]
- Cheng, Y.-N.; Jiang, Z.-H.; Sun, L.-S.; Su, Z.-Y.; Zhang, M.-M.; Li, H.-L. Synthesis of 1, 2, 4-triazole benzoyl arylamine derivatives and their high antifungal activities. Eur. J. Med. Chem. 2020, 200, 112463. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Tian, S.; Yang, X.; Liu, Z. Synthesis methods of 1,2,3-/1,2,4-triazoles: A review. Front. Chem. 2022, 10, 891484. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Tahlan, S.; Narasimhan, B.; Ramasamy, K.; Lim, S.M.; Shah, S.A.A.; Mani, V.; Kakkar, S. Synthesis and biological evaluation of heterocyclic 1,2,4-triazole scaffolds as promising pharmacological agents. BMC Chem. 2022, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, B.F.; Mohamed, H.A.; Farahat, A.A.; Kariuki, B.M.; El-Hiti, G.A. Reactivity of 4-bromoacetyl-1,2,3-triazoles towards amines and phenols: Synthesis and antimicrobial activity of novel heterocycles. Heterocycles 2022, 104, 1601–1613. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Farahat, A.A.; Kariuki, B.M.; El-Hiti, G.A. (E)-1-(5-Methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)ethan-1-one Oxime. Molbank 2023, 2023, M1593. [Google Scholar] [CrossRef]
- Orpen, A.G.; Brammer, L.; Allen, F.H.; Kennard, O.; Watson, D.G.; Taylor, R. Supplement. Tables of bond lengths determined by X-ray and neutron diffraction. Part 2. Organometallic compounds and co-ordination complexes of the d- and f-block metals. J. Chem. Soc. Dalton Trans. 1989, 12, S1–S83. [Google Scholar] [CrossRef]
- Bunev, A.S.; Trushkova, Y.O.; Ostapenko, G.I.; Statsyuk, V.E.; Peregudov, A.S. Synthesis of 4-(1H-1,2,3-triazol-4-yl)-1,3-thiazole-2-amine derivatives. Chem. Heterocycl. Cpds. 2014, 50, 1027–1031. [Google Scholar] [CrossRef]
- Bano, Q.; Tiwari, N.; Giri, S.; Nizamuddin. Synthesis and fungicidal activities of 3-(aryloxymethyl)-6-substistuted 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles. Indian J. Chem. 1992, 31B, 714–718. [Google Scholar]
- Bruker. APEX III; Bruker AXS Inc.: Madison, WI, USA, 2019. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Spek, A.L. Structure Validation in Chemical Crystallography. Acta Crystallogr. 2009, D65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).