4a′-Hydroxy-3′,3′,5,6′,6′,7-hexamethyl-3′,4′,4a′,6′,7′,9a′-hexahydrospiro[indole-3,9′-xanthene]-1′,2,8′(1H,2′H,5′H)-trione
Abstract
:1. Introduction
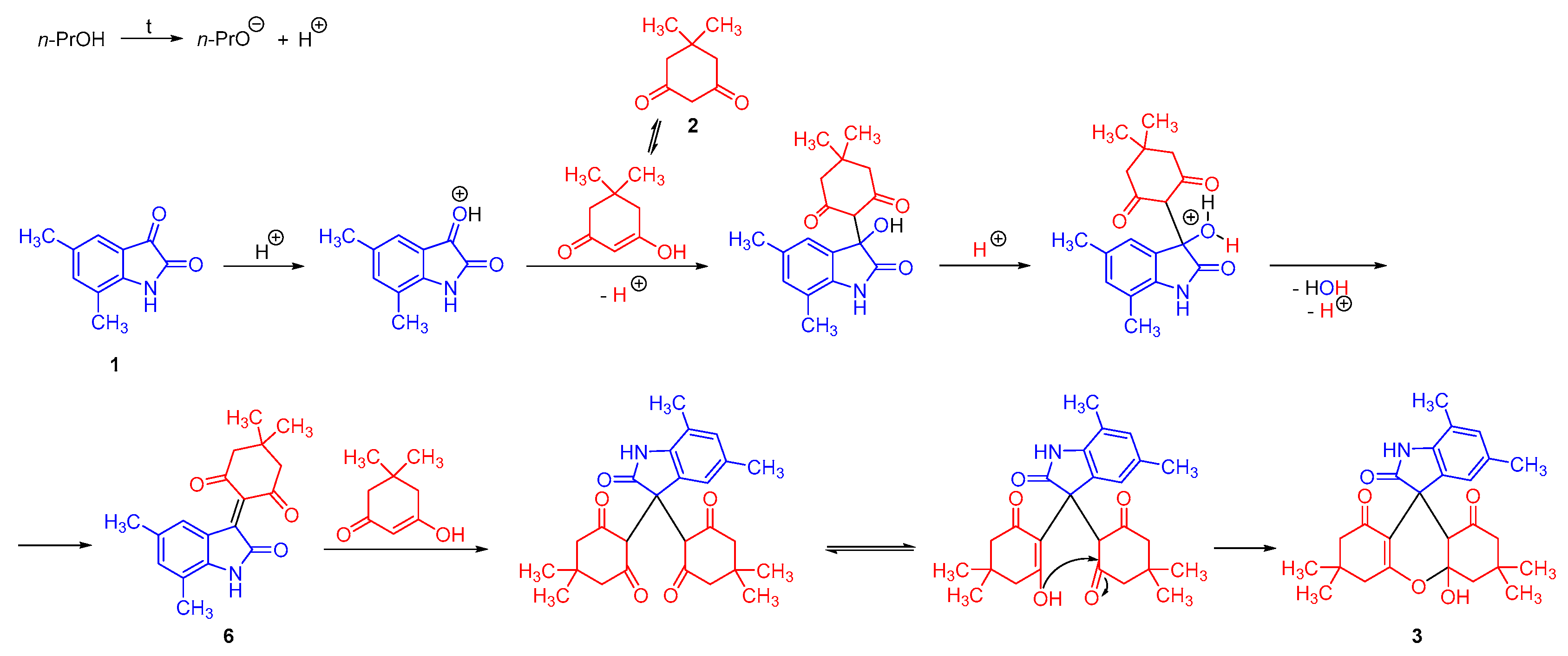
2. Results and Discussion
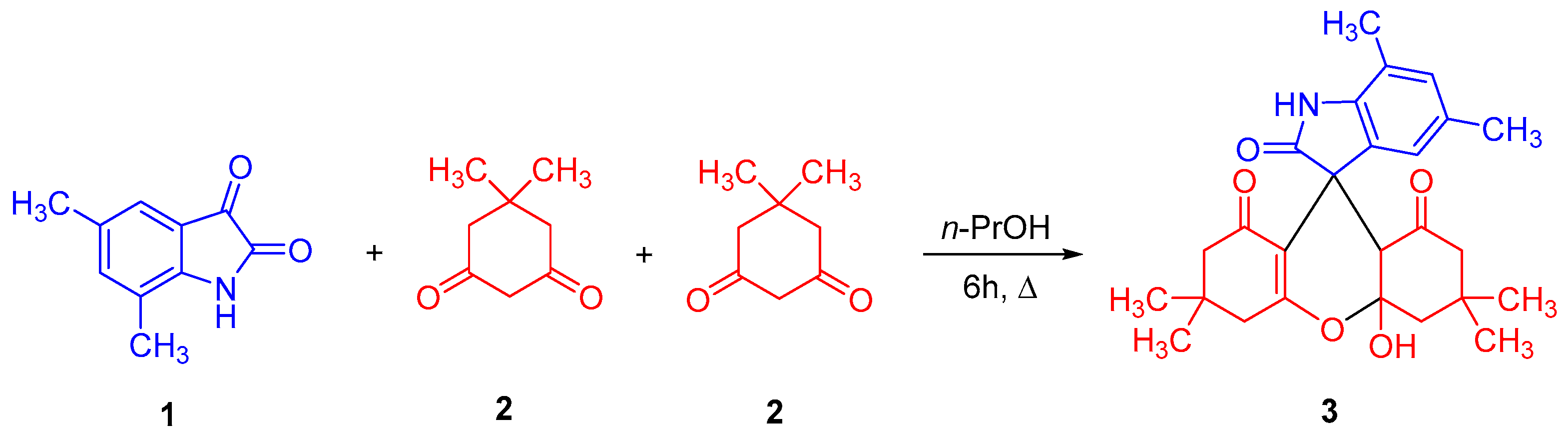
2.1. Synthesis of 4a′-Hydroxy-3′,3′,5,6′,6′,7-Hexamethyl-3′,4′,4a′,6′,7′,9a′-Hexahydrospiro[Indole-3,9′-Xanthene]-1′,2,8′(1H,2′H,5′H)-Trione 3
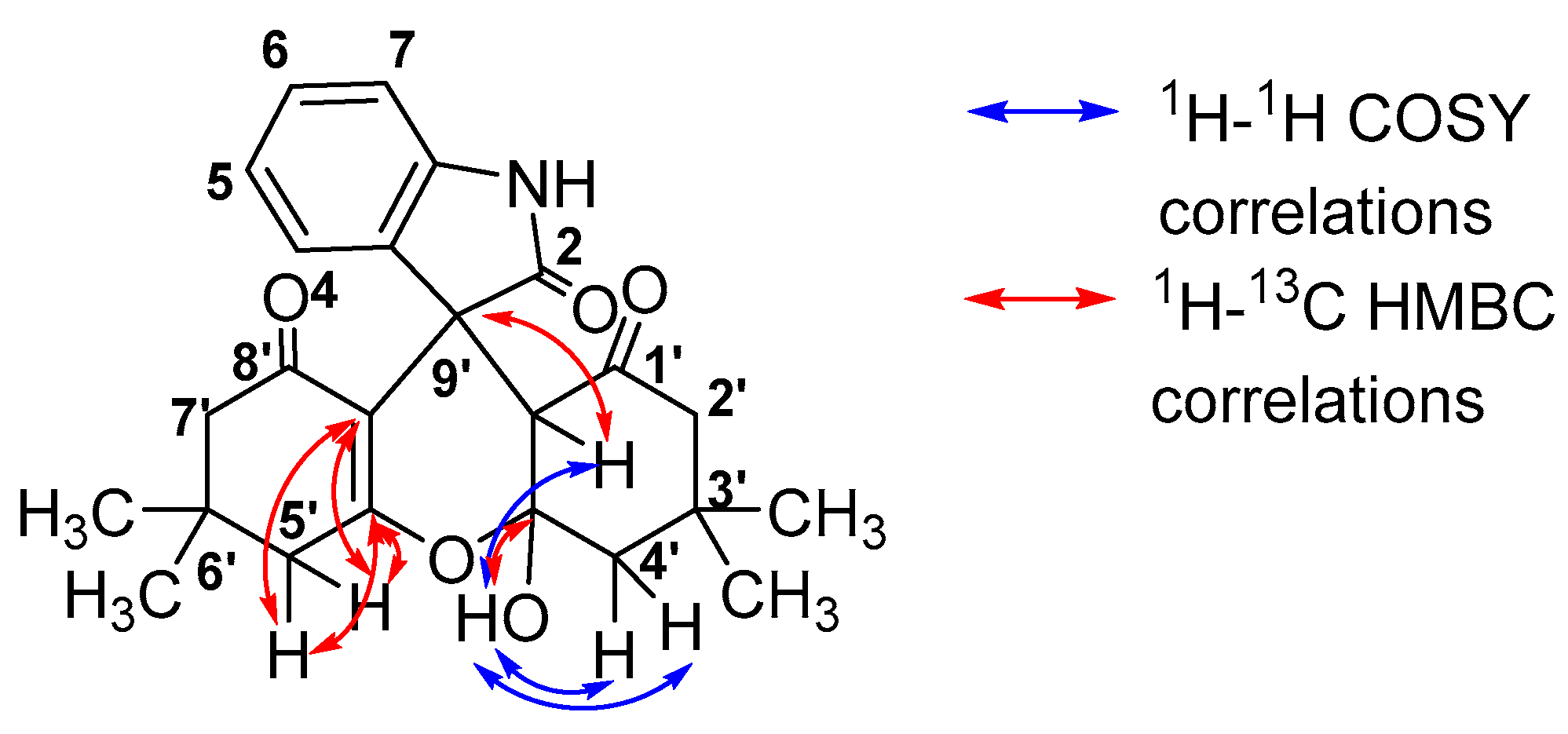
2.2. 2D NMR Study of Compound 5
3. Materials and Methods
3.1. General Methods
3.2. Synthesis of 4a′-Hydroxy-3′,3′,5,6′,6′,7-hexamethyl-3′,4′,4a′,6′,7′,9a′-hexahydrospiro[indole-3,9′-xanthene]-1′,2,8′(1H,2′H,5′H)-trione (3)
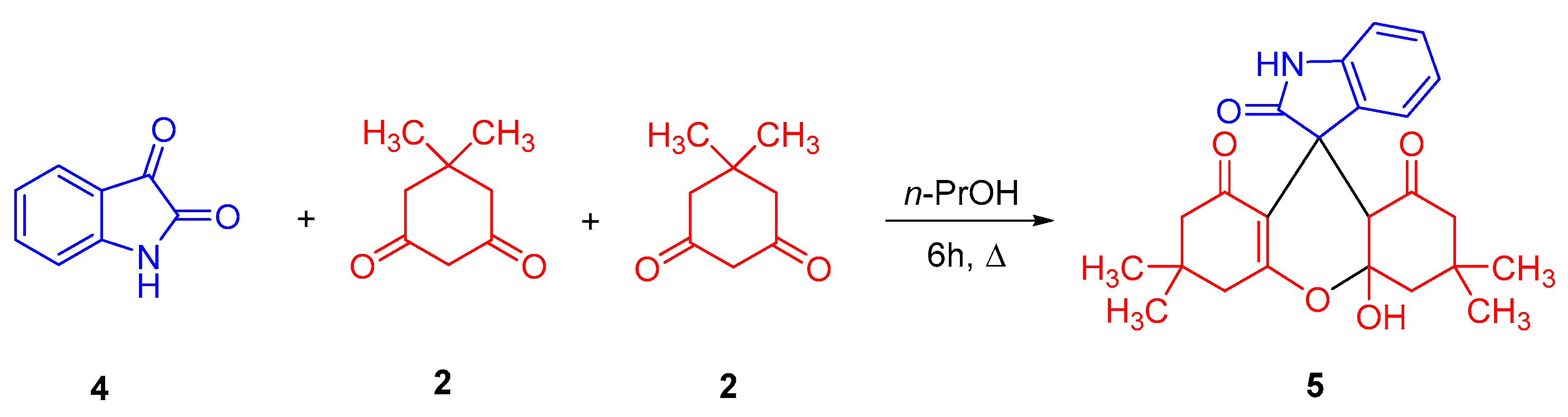
3.3. Synthesis of 4a′-Hydroxy-3′,3′,6′,6′-Tetramethyl-3′,4′,4a′,6′,7′,9a′-Hexahydrospiro[Indole-3,9′-Xanthene]-1′,2,8′(1H,2′H,5′H)-Trione (5)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohammadi, B.; Kazemi, H.; Shafieey, M. Simple pseudo-multicomponent synthesis of 2,6-dicyanoaniline derivatives via reaction between arylidenemalononitriles and malononitrile. Monatsh. Chem. 2014, 145, 1649–1652. [Google Scholar] [CrossRef]
- Flores-Reyes, J.C.; Cotlame-Salinas, V.D.C.; Ibarra, I.A.; González-Zamora, E.; Islas-Jácome, A. Pseudo-multicomponent reactions. RSC Adv. 2023, 13, 16091–16125. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.-C.; Quiroga, J.; Abonia, R.; Rodriguez, J.; Coquerel, Y. Pseudo-Multicomponent Reactions of Arynes with N-Aryl Imines. J. Org. Chem. 2015, 80, 9767–9773. [Google Scholar] [CrossRef]
- Melis, C.; Meleddu, R.; Angeli, A.; Distinto, S.; Bianco, G.; Capasso, C.; Cottiglia, F.; Angius, R.; Supuran, C.T.; Maccioni, E. Isatin: A privileged scaffold for the design of carbonic anhydrase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Rane, R.A.; Karunanidhi, S.; Jain, K.; Shaikh, M.; Hampannavar, G.; Karpoormath, R. A Recent Perspective on Discovery and Development of Diverse Therapeutic Agents Inspired from Isatin Alkaloids. Curr. Top. Med. Chem. 2016, 16, 1262–1289. [Google Scholar] [CrossRef]
- Hou, J.; Jin, K.; Li, J.; Jiang, Y.; Li, X.; Wang, X.; Huang, Y.; Zhang, Y.; Xu, W. LJNK, an indoline-2,3-dione-based aminopeptidase N inhibitor with promising antitumor potency. Anticancer Drugs 2016, 27, 496–507. [Google Scholar] [CrossRef]
- Rana, S.; Blowers, E.C.; Tebbe, C.; Contreras, J.I.; Radhakrishnan, P.; Kizhake, S.; Zhou, T.; Rajule, R.N.; Arnst, J.L.; Munkarah, A.R.; et al. Isatin Derived Spirocyclic Analogues with α-Methylene-γ-butyrolactone as Anticancer Agents: A Structure–Activity Relationship Study. J. Med. Chem. 2016, 59, 5121–5127. [Google Scholar] [CrossRef]
- Meleddu, R.; Distinto, S.; Corona, A.; Bianco, G.; Cannas, V.; Esposito, F.; Artese, A.; Alcaro, S.; Matyus, P.; Bogdan, D.; et al. (3Z)-3-(2-[4-(Aryl)-1,3-thiazol-2-yl]hydrazin-1-ylidene)-2,3-dihydro-1H-indol-2-one derivatives as dual inhibitors of HIV-1 reverse transcriptase. Eur. J. Med. Chem. 2015, 93, 452–460. [Google Scholar] [CrossRef]
- Tavari, M.; Malana, S.F.; Joubert, J. Design, synthesis, biological evaluation and docking studies of sulfonyl isatin derivatives as monoamine oxidase and caspase-3 inhibitors. Med. Chem. Commun. 2016, 7, 1628–1639. [Google Scholar] [CrossRef]
- Akdemir, A.; Güzel-Akdemir, Ö.; Karalı, N.; Supuran, C.T. Isatin analogs as novel inhibitors of Candida spp. β-carbonic anhydrase enzymes. Bioorg. Med. Chem. 2016, 24, 1648–1652. [Google Scholar] [CrossRef]
- Hassan, F.; Azad, I.; Asif, M.; Shukla, D.; Husain, A.; Khan, A.R.; Saquib, M.; Nasibullah, M. Isatin Conjugates as Antibacterial Agents: A Brief Review. Med. Chem. 2023, 19, 413–430. [Google Scholar] [CrossRef]
- Solangi, M.; Kanwal; Khan, K.M.; Chigurupati, S.; Saleem, F.; Qureshi, U.; Ul-Haq, Z.; Jabeen, A.; Felemban, S.G.; Zafar, F.; et al. Isatin thiazoles as antidiabetic: Synthesis, in vitro enzyme inhibitory activities, kinetics, and in silico studies. Arch. Pharm. 2022, 355, e2100481. [Google Scholar] [CrossRef]
- Nikoofar, K.; Yielzoleh, F.M. A concise study on dimedone: A versatile molecule in multi-component reactions, an outlook to the green reaction media. J. Saudi Chem. Soc. 2018, 22, 715–741. [Google Scholar] [CrossRef]
- Singletary, K.; MacDonald, C.; Iovinelli, M.; Fisher, C.; Wallig, M. Effect of the beta-diketones diferuloylmethane (curcumin) and dibenzoylmethane on rat mammary DNA adducts and tumors induced by 7,12-dimethylbenz[a]anthracene. Carcinogenesis 1998, 19, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H.B.; Christensen, S.B.; Kvist, L.P.; Karazmi, A. A simple and efficient separation of the curcumins, the antiprotozoal constituents of Curcuma longa. Planta Med. 2000, 66, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Koiwai, A. Antioxidative β-Diketones in Stigma Lipids of Tobacco. Chem. Biol. Technol. Agric. 1988, 52, 2341–2342. [Google Scholar] [CrossRef]
- Ganguly, N.C.; Mondal, P.; Roy, S. A mild efficient iodine-catalyzed synthesis of novel anticoagulants with 2,8-dioxabicyclo[3.3.1]nonane core. Tetrahedron Lett. 2013, 54, 2386–2390. [Google Scholar] [CrossRef]
- Ghahsare, A.G.; Nazifi, Z.S.; Nazifi, S.M.R. Structure-Bioactivity Relationship Study of Xanthene Derivatives: A Brief Review. Curr. Org. Synth. 2019, 16, 1071–1077. [Google Scholar] [CrossRef]
- Kern, F., Jr.; Almy, T.P.; Stolk, N.J. Effects of certain antispasmodic drugs on the intact human colon, with special reference to banthine (beta-diethylaminoethyl xanthene-9-carboxylate methobromide). Am. J. Med. 1951, 11, 67–74. [Google Scholar] [CrossRef]
- O’Connor, K.D.J.; Mastaglia, F.L. Chapter 32—Drug-Induced Disorders of the Nervous System. In Aminoff’s Neurology and General Medicine, 5th ed.; Aminoff, M.J., Josephson, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 685–711. ISBN 978-0-12-407710-2. [Google Scholar] [CrossRef]
- Gregory Owens, R.; Karram, M.M. Comparative Tolerability of Drug Therapies Used to Treat Incontinence and Enuresis. Drug-Safety 1998, 19, 123–139. [Google Scholar] [CrossRef]
- Höfgen, N.; Beckh, S.; Szelenyi, I.; Bölcskei, P.L. Antiallergic Agents. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH, Ed.; Wiley: Hoboken, NJ, USA, 2003; ISBN 9783527306732. [Google Scholar] [CrossRef]
- Elinson, M.N.; Ilovaisky, A.I.; Dorofeev, A.S.; Merkulova, V.M.; Stepanov, N.O.; Miloserdov, F.M.; Ogibin, Y.N.; Nikishin, G.I. Electrocatalytic multicomponent transformation of cyclic 1,3-diketones, isatins, and malononitrile: Facile and convenient way to functionalized spirocyclic (5,6,7,8-tetrahydro-4H-chromene)-4,3′-oxindole system. Tetrahedron 2007, 63, 10543–10548. [Google Scholar] [CrossRef]
- Elinson, M.N.; Ryzhkov, F.V.; Zaimovskaya, T.A.; Egorov, M.P. Solvent-free multicomponent assembling of isatins, malononitrile, and dimedone: Fast and efficient way to functionalized spirooxindole system. Monatsh. Chem. 2016, 147, 755–760. [Google Scholar] [CrossRef]
- Ryzhkova, Y.E.; Ryzhkov, F.V.; Elinson, M.N. Triethylammonium 2-(3-Hydroxy-2-oxoindolin-3-yl)-5,5-dimethyl-3-oxocyclohex-1-en-1-olate. Molbank 2023, 2023, M1589. [Google Scholar] [CrossRef]
- Elinson, M.N.; Ilovaisky, A.I.; Merkulova, V.M.; Zaimovskaya, T.A.; Nikishin, G.I. Non-Catalytic Thermal Multicomponent Assembling of Isatin, Cyclic CH-Acids and Malononitrile: An Efficient Approach to Spirooxindole Scaffold. Mendeleev Commun. 2012, 22, 143–144. [Google Scholar] [CrossRef]
- Dömling, A.; Wang, W.; Wang, K. Chemistry and Biology of Multicomponent Reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, M.; Mukherjee, R.; Arima, S.; Harigaya, Y. Reaction of Isatins with Active Methylene Compounds on Neutral Alumina: Formation of Knoevenagel Condensates and Other Interesting Products. Heterocycles 2009, 78, 139–149. [Google Scholar] [CrossRef]
- Niknam, K.; Ebrahimpour, A.; Barmak, A.; Mohebbi, G. Synthesis of novel hydroxyspiro[indoline-3,9′-xanthene]trione derivatives using solid acids as catalyst. Monatsh. Chem. 2018, 149, 73–85. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryzhkova, Y.E.; Kalashnikova, V.M.; Ryzhkov, F.V.; Fakhrutdinov, A.N.; Elinson, M.N. 4a′-Hydroxy-3′,3′,5,6′,6′,7-hexamethyl-3′,4′,4a′,6′,7′,9a′-hexahydrospiro[indole-3,9′-xanthene]-1′,2,8′(1H,2′H,5′H)-trione. Molbank 2023, 2023, M1721. https://doi.org/10.3390/M1721
Ryzhkova YE, Kalashnikova VM, Ryzhkov FV, Fakhrutdinov AN, Elinson MN. 4a′-Hydroxy-3′,3′,5,6′,6′,7-hexamethyl-3′,4′,4a′,6′,7′,9a′-hexahydrospiro[indole-3,9′-xanthene]-1′,2,8′(1H,2′H,5′H)-trione. Molbank. 2023; 2023(3):M1721. https://doi.org/10.3390/M1721
Chicago/Turabian StyleRyzhkova, Yuliya E., Varvara M. Kalashnikova, Fedor V. Ryzhkov, Artem N. Fakhrutdinov, and Michail N. Elinson. 2023. "4a′-Hydroxy-3′,3′,5,6′,6′,7-hexamethyl-3′,4′,4a′,6′,7′,9a′-hexahydrospiro[indole-3,9′-xanthene]-1′,2,8′(1H,2′H,5′H)-trione" Molbank 2023, no. 3: M1721. https://doi.org/10.3390/M1721
APA StyleRyzhkova, Y. E., Kalashnikova, V. M., Ryzhkov, F. V., Fakhrutdinov, A. N., & Elinson, M. N. (2023). 4a′-Hydroxy-3′,3′,5,6′,6′,7-hexamethyl-3′,4′,4a′,6′,7′,9a′-hexahydrospiro[indole-3,9′-xanthene]-1′,2,8′(1H,2′H,5′H)-trione. Molbank, 2023(3), M1721. https://doi.org/10.3390/M1721








