Abstract
(E)-3-(2,6-difluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one (1) was synthesized and characterized by 1H and 13C NMR, mass spectrometry, and single-crystal X-ray diffraction. Photolysis (λirr = 300 nm) of chalcone 1 in the crystalline solid state resulted in the stereospecific formation of the syn-head-to-head photodimer (β-truxinic).
1. Introduction
Chalcones (1,3-diaryl-2-propen-1-one) are open-chain flavonoids where two aromatic rings are linked by a three-carbon α,β-unsaturated carbonyl system. They are the biosynthetic precursors of flavones, flavonols, anthocyanins, and isoflavonoids [1,2]. Additionally, chalcones are widely used in organic synthesis as precursors for several heterocyclic compounds [3,4].
Chalcones are a broad class of plant secondary metabolites. In many cases, they actuate in plant defense mechanisms to damage by microorganisms, insects, and herbivores [5,6,7,8,9]. In addition, they are known to possess an antioxidant character to various extents. [10,11] Chalcone derivatives have an overall pharmaceutical value [12,13,14,15,16,17,18] acting as antibiotics [19], antimitotic and cytotoxic [20], anti-tumor [21], anti-HIV [22], antimalarial [23], antitrypanosomal and antileishmanial [24], antifungal [25,26], and anti-inflammatory [27].
Fluorinated chalcones produce inhibition of tubulin polymerization, thus becoming a very efficient antimitotic and, consequently, compromising tumor formation [28]. Fluorinated chalcones are inhibitors of the production of nitric oxide with a calcium-dependent metabolism [29]; therefore, they can act as an antiarthritic [30].
(E)-3-(2,6-difluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one (1) shows antiproliferative activity in the in vitro cytotoxicity against HT29 (human colorectal cancer) and SGC7901 (human gastric cancer cell) [31]. It has also been found that this chalcone can neutralize the toxic effects of lipopolysaccharide (LPS) induced endotoxin [32]. In addition, pharmacological interventions with chalcone 1 have been shown to attenuate cardiac inflammation and fibrosis, resulting in improved cardiac function [33].
Due to the presence of the C=C double bond, chalcones can be present in the E-(trans-) or Z-(cis-) configuration. In addition, the C=C double bond may be oriented trans or cis in relation to the carbonyl group, resulting in s-trans or s-cis conformers. The E-isomer of chalcone is the most thermodynamically stable isomer due to its planarity and lack of torsion caused by steric effects [34,35].
The UV irradiation of trans-chalcones in organic solvents may give four possible stereoisomers through a [2π + 2π] photocycloaddition reaction, i.e., the cyclobutanes syn- head-to-tail (α-truxilic), syn-head-to-head (β-truxinic), anti-head-to-head (δ-truxinic), and anti-head-to-tail (ε-truxilic). In the solid state, cyclobutane formation is strongly dependent on the crystal arrangement [36].
One of the first examples of the photodimerization of chalcones in the solid state is the irradiation of 4-methoxychalcone forming the syn-head-to-head dimer [36]. X-ray analysis of its crystal showed that the shortest contact of C=C groups of two neighboring molecules is 4.2 Å.
In general, the photodimerization reaction of α,β-unsaturated systems, such as cinnamic acid and derivatives [37,38,39], coumarins [40], and chalcones or other α,β-unsaturated ketones [36,41,42,43,44] can lead to the formation of stereoisomeric cyclobutanes when they have intermolecular distances of about 4 Å in the crystalline state, as established by Schmidt’s topological principles [45].
Substitution by halogen atoms in aromatic molecules tends to direct them to distances between C=C double bonds of approximately 4 Å when in the crystalline state, thus allowing more efficient photodimer formation [46,47].
Stereoselective and efficient photodimerization of fluorine-free chalcones and chalcones bearing different numbers of fluorine atoms was recently reported by Shu et al. [48]. It was demonstrated that fluorine can tune the reactivity of photo-induced [2π + 2π] cycloaddition reactions in crystals, leading to the stereospecific formation of the corresponding photodimers. It has also been shown that the introduction of fluorine atoms can lead to an increase in molecular planarity, as well as orienting reactive double bonds so that Schmidt’s criteria for topology-controlled reactions can be met [48].
A systematic study from our group on the photodimerization of fluorinated derivatives of chalcones in the solid state showed that the irradiation of the monosubstituted fluorine derivatives 3- and 4-fluorochalcone resulted in a mixture of anti-head-to-head (δ-truxinic), sin-head-to-tail (α-truxilic), and anti-head-to-tail (ε-truxilic) dimers. On the other hand, irradiation of 3,4- and 3,5-difluorochalcone led to stereoselective formation of the α-truxilic dimer, whereas for 2,3-, 2,5-, and 2,6-difluorochalcone, as well as 2,3,4-trifluorochalcone, the β-truxinic dimer was stereoselectively obtained [49].
In this work, we report the synthesis of (E)-3-(2,6-difluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one (1) and its characterization by 1H and 13C nuclear magnetic resonance (NMR), mass spectrometry, and X-ray crystallography. The photodimerization product formed upon the irradiation of 1 in the crystalline solid state was carefully characterized by 1H and 13C NMR and mass spectrometry.
2. Results and Discussion
2.1. Synthesis and Spectroscopic Characterization of Chalcone 1
(E)-3-(2,6-difluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one (1) was synthesized from a simple aldol condensation reaction in basic medium (aqueous KOH 10% w/v) in 43% yield.
Compound 1 was characterized by 1H and 13C NMR (Figures S1 and S2) and mass spectrometry. Based on the results provided by the NMR spectra, it was possible to correlate the chemical shifts of hydrogen (δH) and carbon (δC) atoms for the chalcone 1. Melting point, spectroscopic and spectrometric data agree with the proposed structures and with literature data [31].
The study of hydrogen shift in the 1H NMR spectrum for chalcones generally follows what is predicted by the NMR theory regarding the location of the chemical shifts of each proton present in the molecule. The presence of the methoxy group on ring A gives the hydrogen located in the 3′ and 5′ positions a high degree of symmetry, leading to the same chemical environment, with absorption between 6.94 and 7.04 ppm. The proximity of the methoxy group causes these hydrogens (3′ and 5′) to be in a high field. Their proximity with the 2′ and 6′ hydrogens causes the signal feature to appear as a doublet. The same can be said for the sign of the hydrogens at the 2′ and 6′ positions. In this case, however, the presence of the carbonyl group causes its signal (also a doublet) to be in a low field (between 7.99 and 8.08 ppm) due to the deprotection imposed by the ketone carbonyl. Noteworthy are the signals for the hydrogens of the B ring that are close to fluorine atoms, shifted to a low field in relation to the other hydrogens of this ring.
The main information extracted from the proton NMR spectrum (Figure S2) concerns the configuration presented by chalcone 1. It is well known that conveniently substituted α,β-unsaturated ketones can show either an E or Z configuration, with the former being thermodynamically more stable. The doublets in the region of 7.95–7.86 ppm (J = 16.27 Hz) and 7.86–7.78 ppm (J = 16.27 Hz) in the 1H NMR spectrum clearly indicate an E configuration for 1. It is important to note that, in the case of chalcones with a Z-configuration, the olefinic protons appear as doublets in the region of 7.0 (J~8.0 Hz) and 6.6 ppm (J~8.0 Hz) [32]. In the case of 13C NMR, the absorptions of interest correspond to the carbon atom of the methoxyl group (55.48 ppm) and the carbonyl carbon (188.60) (Figure S3).
2.2. Characterization of Chalcone 1 by X-ray Crystallography
The evaporative crystallization process on the Pyrex test tube surface (see Materials and Methods) can result in defective crystal structures. Therefore, for the characterization of 1 by X-ray crystallography, it was necessary to prepare monocrystals. The crystallographic structure for 1 is shown in Figure 1, with selected intramolecular interactions shown in Figure 1b. The X-ray analysis of (E)-3-(2,6-difluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one (1) reveals an almost planar structure with values of the angles 1 and 2 of −1.26 and 1.04°, respectively (Figure 1a).
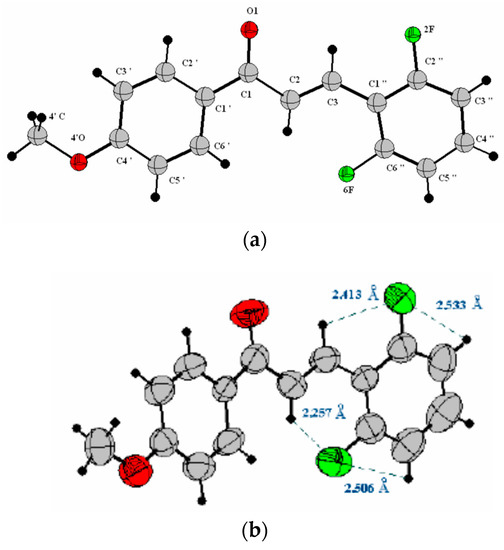
Figure 1.
(a) Molecular structure of (E)-3-(2,6-difluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one (1) with crystallographic numbering employing ORTEP. Selected torsion angles (°): O1-C1-C2-C3 11.50(8), C1-C2-C3-C1′ 11.03(5), C1-C2-C1′-C2′ -1.26(5), C1-C2-C1′-C6′ 1.04(7), C2-C3-C1″-C6″ 10.82(8), C1″-C3-C2-C1 2.11(1). (b) Intramolecular interactions between fluorine atoms and neighboring hydrogens for 1.
Figure 1b reveals a non-covalent interaction between the hydrogens of the olefinic residue and the fluorine atoms present in positions 2 and 6 of the B ring that (E)-3-(2,6-difluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one (1) crystals. This interaction gives great planarity to the system, resulting in a value for the dihedral angle C1″-C3-C2-C1 of 2.11°. This planarity can be confirmed by considering the angle O1-C1-C2-C3 of 11.50° between the carbonyl group and the conjugated double bond. For this chalcone, the distance between the olefinic groups is 4.035(6) Å (Figure 2), from which one can predict, following Schmidt’s topological rules [45], the stereospecific formation of the syn-head-to-head (β-truxinic) dimer of 1 in the crystalline state upon its photochemical irradiation (see below). Topological rules state that reactions in crystals take place with a minimum of atomic and molecular motion. Therefore, for a photocycloaddition reaction [2π + 2π] to occur, it is of paramount importance that the chalcone double bonds are parallel and at a distance d < 4.2 Å, so that the coupling between orbitals is effective [45].
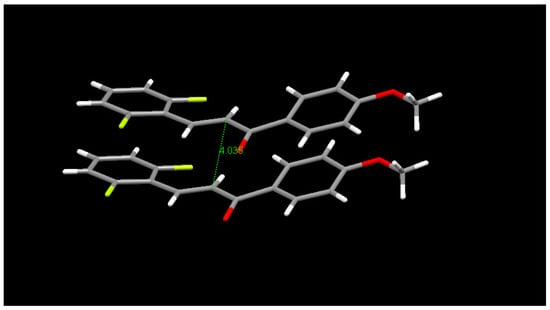
Figure 2.
Intermolecular interaction for (E)-3-(2,6-difluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one (1).
2.3. Photochemical Reaction
Photolysis of chalcones in solution or in solid state can yield a mixture of isomeric dimers through a [2π + 2π] cycloaddition reaction [50]. It is well known that the intersystem crossing quantum yield in chalcones is close to unity. Consequently, the triplet excited state of chalcones, which has a radical characteristic, must be the transient involved in the photodimerization process [2π + 2π] in these compounds. Thus, the stereospecificity observed in forming a photodimer should not be described as a concerted reaction, but as having a topological control.
For (E)-3-(2,6-difluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one (1) the structure for the photodimers possibly formed in this reaction is shown in Figure 3.
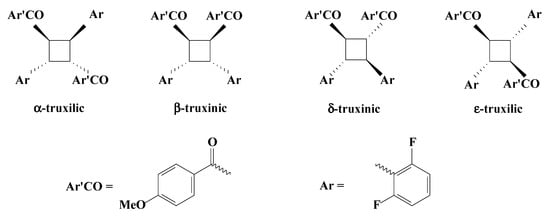
Figure 3.
Possible cyclobutanes that can be formed in the photodimerization reaction of chalcone 1.
Irradiation (λ = 300 nm) of (E)-3-(2,6-difluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one (1) in the crystalline solid state efficiently led to the stereospecific formation of its β-truxinic photodimer, with its characterization based on multiplicity patterns of signals in 1H and 13C NMR spectra and by mass spectrometry. The efficiency in forming photodimers was demonstrated by experiments employing UV-visible spectroscopy. The representative absorption spectrum, in dichloromethane, for the photolysis of 1 clearly shows that the chalcone was consumed entirely after 240 min of irradiation (Figure 4). In this figure, it can be clearly observed that the starting material’s absorption at 325 nm, corresponding to a ππ* transition for the chalcone, is substituted for a much less intense, blue-shifted band, showing a maximum at about 275 nm. This new absorption could be associated with the formation of photodimers, since, as they do not have a conjugated system, they must be absorbed at shorter wavelengths and with a lower molar extinction coefficient than the starting monomeric chalcone. Furthermore, it is worth emphasizing the stability of the photodimers formed, since even after 600 min of irradiation, no change in the spectrum was observed compared to that obtained at 240 min.
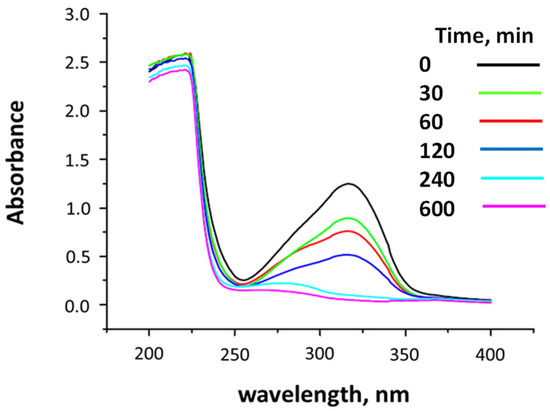
Figure 4.
UV-visible absorption spectra for (E)-3-(2,6-difluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one (1), in dichloromethane, at different irradiation times.
The 1H NMR spectrum obtained after irradiation (λ = 300 nm) of 1 (Figure 5) revealed that in addition to the presence of aromatic hydrogens (multiplet from 6.5 to 8.1 ppm) and methyl hydrogens of the methoxyl group (singlet, 3.87 ppm), the appearance of new protons with chemical shift at 5.29/5.25 and 4.86/4.82 ppm (J = 6.69 Hz) was observed. These absorptions correspond to cyclobutane protons and appear as an AA′BB′-type system, due to the presence of a plane of symmetry, which is characteristic of a β-truxinic-type dimer. The multiplicity pattern for this system is presented as a multiplet, similar to a double-triplet. These results agree with those obtained by X-ray crystallography (Figure 2), from which we could predict the stereospecific formation of a syn-head-to-head (β-truxinic) dimer on irradiation of 1 in the crystalline state.
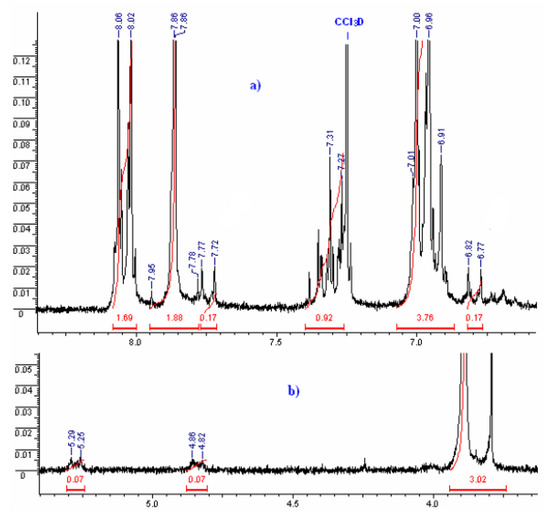
Figure 5.
1H NMR for the product formed on irradiation (λ = 300 nm) of (E)-3-(2,6-difluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one in the crystalline solid state. (a) 6.60–8.35 ppm; (b) 3.65–5.40 ppm. Marked in red are he corresponding peak integrations.
The structure of the photodimer resulting from the irradiation of 1 in the crystalline solid state was confirmed by gas chromatography coupled with mass spectrometry (Figure S3), with the results showing the stereospecific formation of the syn-head-to-head photodimer (β-truxinic) in quantitative yields, as explained below. It is important to note that although mass spectrometry is considered a powerful tool for structural elucidation, under the conditions of the present experiment (Electron Impact—EI, 70 eV), this technique was not conclusive for two reasons. The first is that generally the molecular ion intensity of cyclobutanes is very low (<6%) due to the presence of efficient symmetrical fragmentation modes (Figure 6). Secondly, cyclobutanes formed upon the irradiation of aromatic α,β-unsaturated ketones always have two benzoyl groups in their structure that are easily fragmented, resulting in the formation of the ArCO+ ion (m/z = 135), which has high intensity due to its stability.
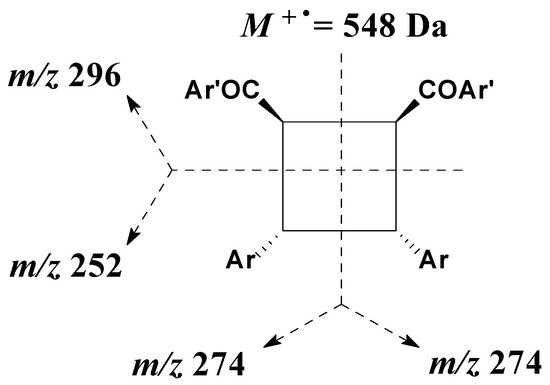
Figure 6.
Symmetrical fragmentation modes of a cyclobutane ring in the mass spectrometer (EI 70 eV).
In the mass spectrum for the photodimer generated in the irradiation of chalcone 1 in the crystalline solid state the molecular ion (M+. at 548 Da) was not observed, nor fragments corresponding to the symmetrical cleavage of the cyclobutane moiety at m/z 252, 274, or 296 (Figure 6). The base peak observed corresponds to the fragment at m/z 275, which can be formed directly from the fragmentation of the molecular ion (M+. 548 Da).
Despite the difficulty in identifying the resulting photodimers in cycloaddition reactions employing mass spectrometry, it can be suggested that, specifically for the syn head-to-head photodimer (β-truxinic) formed in the photodimerization reaction of 1, the presence of the extremely important fragment at m/z 275 indicates a fragmentation mode known as MacLafferty rearrangement that can unequivocally confirm the presence of the β-truxinic structure. This is because only the syn head-to-head photodimer has a stereochemistry in which the carbonyl group and the γ-H are on the same side of the cyclobutane plane, an essential steric requirement for the rearrangement to occur [51]. The mechanism for fragment formation at m/z 275+ via a MacLafferty rearrangement is described in Figure 7.
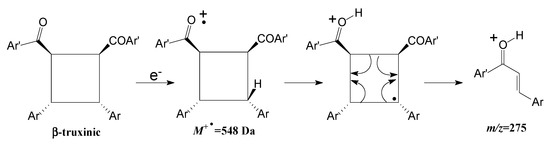
Figure 7.
MacLafferty rearrangement for the molecular ion M+. 548 Da of the β-truxinic photodimer, formed from the irradiation (λ = 300 nm) of chalcone 1 in the crystalline solid state.
3. Materials and Methods
Ethanol (spectrophotometric grade), dichloromethane (spectrophotometric grade), potassium and sodium hydroxide were purchased from Grupo Química, Brazil. 2,6-Difluorobenzaldehyde and 4′-methoxyacetophenone were purchased from Aldrich Chemical and used as received.
1H and 13C Nuclear Magnetic Resonance spectra were obtained using either a Bruker model AC 200 (1H: 200 MHz; 13C: 50.3 MHz) or a BRUKER DMX 400 MHz spectrometer with a BBI probe (broadband). Tetramethylsilane in CDCl3 was used as an internal reference.
The mass spectrum was obtained by coupling gas chromatography–mass spectrometry (GC–MS) on a Hewlett-Packard model 5995 mass spectrometer using a 12 m HP–5 fused silica capillary column. The mass spectra were recorded with the mass spectrometer operating at 70 eV.
The spectra in the ultraviolet–visible (UV-vis) region were obtained in a Varian model DMS–80 spectrophotometer, using CH2Cl2 as a solvent and a quartz cell with an optical path of 1.0 cm.
Melting point was determined using a Kofler-type apparatus and were not corrected.
The crystallographic structures were determined using the SUPERGUI diffractometer (NONIUS-KAPPA-CCD, Bruker 2004) employing the COLLECT program of the Nonius Kappa CCD software package (1998).
(E)-3-(2,6-difluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one synthesis. The method used for the synthesis was a variation of the one used by Koller and Chadwell [52], employing aldol condensation in a basic medium. In an ice bath (5 °C), to a 50 mL flask equipped with a magnetic stirrer containing an ethanol/water mixture (9:6) and potassium hydroxide to obtain a concentration of 10% w/v (0. 1 g/mL), the total amount of 2,6-difluorobenzaldehyde (500 mg, 0.35 mmol) was added, followed by half the mass of 4′-methoxyacetophenone (250 mg, 0.165 mmol). After the start of the reaction, under constant stirring, the remainder of the 4′-methoxyacetophenone (250 mg, 0.165 mmol) was added, and the reaction continued until a slightly yellow precipitate was obtained. The reaction mixture was then filtered, and the solid residue obtained was washed with ice-cold water until neutral pH (measured on litmus paper). The solid was filtered under reduced pressure and kept away from light. The chalcone was recrystallized successively from EtOH/H2O, and the spectroscopic and spectrometric data listed below agree with the proposed structure and with literature data [31]. To facilitate 1H and 13C NMR interpretation, chalcone numbering is shown in Figure 8.
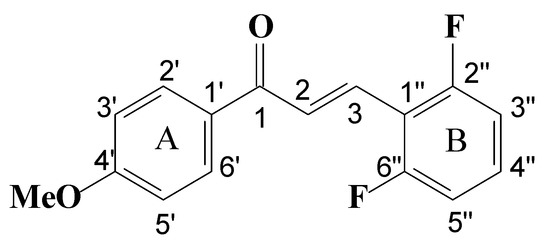
Figure 8.
Chalcone numbering for 1H and 13C NMR interpretation.
(E)-3-(2,6-difluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one (1)—NMR 1H δ (ppm): 8.06–8.02 (d; 2H, H2′, H6′; J = 9.08 Hz); 7.95–7.86 (d; 1H, H3, J = 16.27 Hz); 7.86–7.78 (d; 1H, H2, J = 16.27 Hz); 7.31–7.27 (m; 1H, H4″); 7.01–6.91 (m; 2H, H3″, H5″); 7.00–6.96 (d; 2H, H3′, H5′; J = 9.08 Hz); 3.87 (s, 3H, MeO). 13C d (ppm): 188.60 (C1); 127.59 (C2); 130.68 (C3); 111.59 (C1″); 164.55 (C2″); 112.12 (C3″); 129.69 (C4″); 112.12 (C5″); 164.55 (C6″); 129.69 (C1′); 130.94 (C2′C6′); 113.88 (C3′C5′); 163.73 (C4′); 55.48 (OCH3). MS (m/z) %: 274 (M+., 100); 273 (20); 255 (34); 245 (13); 214 (9); 196 (11); 167 (44); 139 (27); 135 (56); 119 (33); 107 (78). Yield: 43%. m.p. = 88–89 °C.
3.1. Photodimerization Reaction
Crystals of 1 were prepared by evaporation of a solution of about 1.0 molL−1 in dichloromethane, ethanol, or acetone on the inner wall of a Quartz test tube. The resulting crystals were filtered, lyophilized, and kept protected from light. The monomorphic crystals of 1 thus obtained were degassed in Quartz test tubes using Argon, sealed with a rubber septum, and irradiated in the ultraviolet region (λ = 300 nm) for different time periods (from 0 to 4 h). The irradiation system consisted of a merry-go-round and a Hanovia medium-pressure mercury lamp (450 W) centered in a quartz jacket and refrigerated by a distilled water circulating bath. The resulting single crystals were analyzed by 1H NMR and mass spectrometry. The photochemical reactivity of the chalcone is represented by the results of consumption of its E isomer against irradiation time, as determined by UV-visible spectroscopy.
3.2. X-ray Crystallography
Monocrystals of 1 were obtained through the technique of slow evaporation of the solvent [53]. This technique consists of adding approximately 10 mg of chalcone to a Pyrex test tube to which 6 mL of ethanol has been added. The solution was sonicated until complete solubilization, at which point the tube was closed with a rubber septum. A hypodermic needle was introduced in the center of the septum so that it would not touch the solution that was protected from the light until the evaporation of the solvent and the appearance of crystals (between three to four days) [53].
Data collection: NONIUS 1998 COLLECT; cell refinement: PHICHI [54]; data reduction: DIRAX [55]; program(s) used to solve structure: SHELXS97; program(s) used to refine structure: SHELXL97; molecular graphics: ORTEP-3 for Windows [56]; software used to prepare material for publication: WinGX [57].
Crystal Data for (E)-3-(2,6-difluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one (1). C16H12O2F2 (M = 274.2788 g/mol): monoclinic, space group P21/c, a = 4.0348(8) Å, b = 12.120(2) Å, c = 27.223(5) Å, a = 90°, b = 90.60(3)°, g = 90°, V = 1331.18(5) Å3, Z = 4, T = 293(2) K, μ(MoKα) = 0.11 mm−1, Dcalc = 1.368 g/cm3, Refinement R[F2 > 2σ(F2)] = 0.1175, wR(F2) = 0.1732, S = 1.058, 1510 measured reflections, 2352 unique (Rint = 0.061, Rsigma = 0.056) which were used in all calculations.
In the Independent Atom Model (IAM) approach, the scattering factors were taken from international tables of crystallography, being based on Hartree–Fock electron densities of isolated atoms of average spherical morphology. Thus, for all hydrogen atoms attached to sp2 carbons, both aromatic and olefinic, the distances were fixed at 0.93 Å, with an isotropic parameter 20% greater than the equivalent isotropic shift parameter for the carbon atom, i.e., [Uiso(H) = 1.2 Ueq(Csp2)]. For the carbon atom with sp3 hybridization, an idealized distance for bonding with the hydrogen atoms of 0.960 Å was used. The phenyl groups were refined as rigid groups.
CCDC code 2220592 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures.
4. Conclusions
The X-ray crystallography study for (E)-3-(2,6-difluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one (1) showed that it has an almost planar structure. Photolysis (λ = 300 nm) of 1 in the crystalline solid state resulted in the stereospecific formation of its β-truxinic dimer. The reaction was highly efficient, as shown from UV-visible results. The unequivocal characterization of this photodimer was evidenced by the presence in their mass spectrum of a fragment at m/z 275. This fragmentation occurs due to the involvement of a MacLafferty rearrangement that is only possible for this type of isomer. The stereospecific formation of the syn-head-to-head (β-truxinic) dimer on irradiation of (E)-3-(2,6-difluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one (1) in the crystalline solid state follows Schmidt’s topological rules.
Supplementary Materials
The following spectra are available online. Figures S1 and S2: 1H- and 13C-NMR spectra for (E)-3-(2,6-difluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one (1). Figure S3: mass spectrum for the photodimer formed on irradiation (λ = 300 nm) of 1.
Author Contributions
Conceptualization, J.C.N.-F.; synthesis and molecule characterization, A.F.d.P. and D.C.-S.; photochemical reactions: A.F.d.P.; spectroscopic and crystallographic analysis, A.F.d.P. and D.C.-S.; writing—review and editing, J.C.N.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Brazilian agencies: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data and in the writing of the manuscript or in the decision to publish the results.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge Jairo Bordinhão (in memorian) and Lorenzo Visentin, from the Institute of Chemistry at Universidade Federal do Rio de Janeiro (Brazil), for the crystallographic data acquisition.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ávila, H.P.; Smânia, E.F.A.; Monache, F.D.; Smânia Júnior, A. Structure–activity relationship of antibacterial chalcones. Bioorg. Med. Chem. 2008, 16, 9790–9794. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.N.A.; Jasamai, M.; Jantan, I.; Ahmad, W. Review of methods and various catalysts used for chalcone synthesis. Mini-Rev. Org. Chem. 2013, 10, 73–83. [Google Scholar] [CrossRef]
- Chopra, P.K.P.G. Chalcones: A brief review. Int. J. Res. Eng. Appl. Sci. 2016, 6, 173–185. [Google Scholar]
- Wang, S.; Yu, G.; Lu, J.; Xiao, K.; Hu, Y.; Hu, H. A Regioselective Tandem reaction between chalcones and 2-acetamido-acetamide promoted by Cs2CO3 for the preparation of 33-unsubstituted 2-pyridones. Synthesis 2003, 487, 487–490. [Google Scholar]
- Chawla, H.M.; Chibber, S.S.; Chakrabarty, K. Daphnetin from Euphorbia dracunculoides fruits. Ind. J. Pharm. Sci. 1980, 42, 138–139. [Google Scholar]
- Chawla, H.M.; Chaudhuri, K. Chemical components of Cassia javanica leaves. Ind. J. Pharm. Sci. 1985, 47, 172–173. [Google Scholar]
- Rajnarayana, K.; Sripal Reddy, M.; Chaluvadi, M.R.; Krishna, D.R. Biflavonoids Classification, Pharmacological, Biochemical Effects and Therapheutic Potential. Indian J. Pharmacol. 2001, 33, 2–16. [Google Scholar]
- Matsushima, R.; Hirao, I. Photocyclization of 2′-Hydroxychalcones to 4-Flavanones. Bull. Chem. Soc. Jpn. 1980, 53, 518–520. [Google Scholar] [CrossRef]
- Miquel, J.F. Chimie Organic-Isomerie cis-trans des Styrylcetones et 2′hidroxychalcones. Compt. Rend. Hebd. Séances Acad. Sci. 1962, 254, 4479. [Google Scholar]
- Cioffi, G.; Escobar, L.M.; Braca, A.; Tommasi, N.D. Antioxidant Chalcone Glycosides and Flavanones from Maclura (Chlorophora) tinctoria. J. Nat. Prod. 2003, 66, 1061–1064. [Google Scholar] [CrossRef]
- Nakamura, Y.; Watanabe, S.; Miyake, N.; Kohno, H.; Osawa, T. Dihydrochalcones: Evaluation as novel radical scavenging antioxidants. J. Agric. Food Chem. 2003, 51, 3309–3312. [Google Scholar] [CrossRef] [PubMed]
- Herencia, F.; Ferrandiz, M.L.; Ubeda, A.; Guille, I.; Dominguez, J.N.; Charris, J.E.; Gricela, M.; Lobo, M.; Alcaraz, J. Novel anti-inflammatory chalcone derivatives inhibit the induction of nitric oxide synthase and cyclooxygenase-2 in mouse peritoneal macrophages. FEBS Lett. 1999, 453, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.B.; Su, C.-R.; Chiou, W.-F.; Liu, Y.-N.; Chen, R.Y.-H.; Bastow, K.F.; Lee, K.-H.; Wu, T.-S. Design, synthesis, and biological evaluation of Mannich bases of heterocyclic chalcone analogs as cytotoxic agents. Bioorg. Med. Chem. Lett. 2008, 16, 7358–7370. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, J.R.; Elias, D.W.; Beazely, M.A.; Kandepu, N.M. Bioactivities of chalcones. Curr. Med. Chem. 1999, 6, 1125–1149. [Google Scholar] [CrossRef] [PubMed]
- Go, M.L.; Wu, X.; Liu, X.L. Chalcones: An update on cytotoxic and chemoprotective properties. Curr. Med. Chem. 2005, 12, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Meng, C.Q.; Sikorski, J.A. Recent advances in therapeutic chalcones. Expert Opin. Ther. Pat. 2004, 14, 1669–1691. [Google Scholar] [CrossRef]
- Yit, C.C.; Das, N.P. Cytotoxic effect of butein on human colon adenocarcinoma cell proliferation. Cancer Lett. 1994, 82, 65–72. [Google Scholar] [CrossRef]
- Ramanathan, R.; Tan, C.H.; Das, N.P. Cytotoxic effect of plant polyphenols and fat-soluble vitamins on malignant human cultured cells. Cancer Lett. 1992, 62, 217–224. [Google Scholar] [CrossRef]
- Bowden, K.; Dalpozzo, A.D.; Duah, C.K. Structure-Activity. Part 5. Antibacterial Activity of Substituted (E)-3-(4-Phenylbenzoyl)acrylic Acids, Chalcones, 2- hydroxychalcones and-α-Bromochalcones; Addition of Cysteine to Substituted 3-Benzoylacrylic Acids and Related Compounds. J. Chem. Res. 1990, 377. [Google Scholar]
- Wilhelm, A.; Bonnet, S.L.; Twigge, L.; Rarova, L.; Stenclova, T.; Visser, H.G.; Schutte-Smith, M. Synthesis, characterization and cytotoxic evaluation of chalcone derivatives. J. Mol. Struct. 2022, 1251, 132001. [Google Scholar] [CrossRef]
- Iwata, S.; Nishino, T.; Nagata, N.; Satomi, Y.; Nishino, H.; Shibata, S. Atitumorigenic Activitties of Chalcones. I. Inhibitory Effects of Chalcone Derivatives on Pi-Incorporation into phospholipdis of HeLa Cells Promoted by 12-O-Tetradecanoyl-phorbol 13-Acetate (TPA). Biol. Pharm. Bull. 1995, 18, 1710–1713. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Q.; Chen, K.; Shi, Q.; Kilkuskie, R.E.; Cheng, Y.C.; Lee, K.H. Anti-aids agents, 10. Acacetin-7-O-β-D-galactopyranoside, an anti-HIV principle from Chrysanthemum morifolium and a structure-activity correlation with some related flavonoids. J. Nat. Prod. 1994, 57, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Kenyon, G.L.; Cohen, F.E.; Chem, X.; Gong, B.; Miller, R.E.; Nuzum, E.O.; Resenthal, P.J.; McKerrow, J.H. In Vitro Antimalarial Activity of Chalcones and Their Derivatives. J. Med. Chem. 1995, 38, 5031–5037. [Google Scholar] [CrossRef] [PubMed]
- Boeck, P.; Bandeira-Falcão, C.A.; Leal, P.C.; Yunes, R.A.; Filho, V.C.; Torres-Santos, E.C. Synthesis of chalcone analogues with increased antileishmanial activity. Bioorg. Med. Chem. 2006, 14, 1538–1545. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, F.; Guzela, M.; Rodrigues, A.T.; Correa, R.; Mangrich, I.E.; Steindel, M.; Grisard, E.C.; Assreuy, J.; Calixto, J.B.; Santos, A.R.S. Trypanocidal and Leishmanicidal Properties of Substitution-Containing Chalcones. Antimicrob. Agents Chemother. 2003, 47, 1449–1451. [Google Scholar] [CrossRef]
- López, S.N.; Castelli, M.A.V.; Zacchino, S.A.; Dominguez, J.N.; Lobo, G.; Charris-Charris, J.; Cortes, J.C.G.; Ribas, J.C.; Devia, C.; Rodriguez, A.M.; et al. In Vitro Antifungal Evaluation and Structure–Activity Relationships of a New Series of Chalcone Derivatives and Synthetic Analogues, with Inhibitory Properties against Polymers of the Fungal Cell Wall. Bioorg. Med. Chem. 2001, 9, 1999–2013. [Google Scholar] [CrossRef]
- Alias, Y.; Awang, K.; Hadi, A.; Thoison, O.; Sevent, T.; Pais, M. An Antimitotic and Cytotoxic chalcone from Fissitigma Lanuginosum. J. Nat. Prod. 1995, 58, 1160. [Google Scholar] [CrossRef]
- Lawrence, N.J.; Patterson, R.P.; Ooi, L.L.; Cook, D.; Ducki, S. Effects of α- substitutions on structure and biological activity of anticancer chalcones. Bioorg. Med. Chem. Lett. 2006, 16, 5844–5848. [Google Scholar] [CrossRef]
- Rojas, J.; Paya, M.; Dominguez, J.N.; Ferrandiz, L.M. The Synthesis and Effect of Fluorinated Chalcone Derivatives on Nitric Oxide Production. Bioorg. Med. Chem. Lett. 2002, 12, 1951–1954. [Google Scholar] [CrossRef]
- Rojas, J.; Paya, M.; Dominguez, J.N.; Ferrandiz, L.M. ttCH, a selective inhibitor of inducible nitric oxide synthase expression with antiarthritic properties. Eur. J. Pharmacol. 2003, 465, 183–189. [Google Scholar] [CrossRef]
- Wu, J.; Wang, C.; Cai, Y.; Peng, J.; Liang, D.; Zhao, Y.; Yang, S.; Li, X.; Wu, X.; Liang, G. Synthesis and crystal structure of chalcones as well as on cytotoxicity and antibacterial properties. Med. Chem. Res. 2012, 21, 444–452. [Google Scholar] [CrossRef]
- Wu, P.; Yan, H.; Qi, J.; Jia, W.; Zhang, W.; Yao, D.; Ding, C.; Zhang, Y.; Chen, M.; Cai, X. L6H9 attenuates LPS-induced acute lung injury in rats through targeting MD2. Drug Dev. Res. 2020, 81, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Sumneang, N.; Apaijai, N.; Chattipakorn, S.C.; Chattipakorn, N. Myeloid differentiation factor 2 in the heart: Bench to bedside evidence for potential clinical benefits? Pharmacol. Res. 2021, 163, 105239. [Google Scholar] [CrossRef] [PubMed]
- Aksöz, B.E.; Ertan, R. Chemical and structural properties of chalcones I. J. Pharm. Sci. 2011, 36, 223–242. [Google Scholar]
- Aksöz, B.E.; Ertan, R. Chemical and structural properties of chalcones II. J. Pharm. Sci. 2012, 37, 205–216. [Google Scholar]
- Rabinovich, D.; Schmidt, G.M.J. Topochemistry. Part XXIX. Crystal and molecular structures of p-methoxychalcone. J. Chem. Soc. B 1970, 6–10. [Google Scholar] [CrossRef]
- Green, B.S.; Schmidt, G.M.J. Topochemically-controlled solid-state photodimerization to a tricyclo [6.2.0.0.3,6]-decane derivative. Tetrahedron Lett. 1970, 11, 4249–4256. [Google Scholar] [CrossRef]
- Abdelmoty, I.; Buchholz, V.; Di, L.; Guo, C.; Kowitz, K.; Enkelmann, V.; Wegner, G.; Foxman, B.M. Polymorphism of Cinnamic and r-Truxillic Acids: New Additions to an Old Story. Cryst. Growth Des. 2005, 5, 2210–2217. [Google Scholar] [CrossRef]
- Atkinson, S.D.M.; Almond, M.J.; Hollins, P.; Jenkins, S.L. The photodimerisation of the α-and β-forms of trans-cinnamic acid: A study of single crystals by vibrational microspectroscopy. Spectrochim. Acta A 2003, 59, 629–635. [Google Scholar] [CrossRef]
- Gnanaguru, K.; Ramasubbu, N.; Venkatesan, K.; Ramamurthy, V. A study on the photochemical dimerization of coumarins in the solid state. J. Org. Chem. 1985, 50, 2337–2346. [Google Scholar] [CrossRef]
- Dihurjati, M.S.K.; Sarma, J.A.R.P.; Desiraju, G.R. Unusual [2 + 2] topochemical cycloadditions of 3-cyano- and 4-cyano-cinnamic acids: Temperature dependent solid state photochemical reactions. J. Chem. Soc. Chem. Commun. 1991, 1702–1703. [Google Scholar] [CrossRef]
- Caccamese, S.; McMillan, J.A.; Montaudo, G. Revision of the stereochemical assignment of a cyclobutane derivative from chalcone photodimerization via X-ray diffraction analysis. J. Org. Chem. 1978, 43, 2703–2704. [Google Scholar] [CrossRef]
- Montaudo, G.; Caccamese, S. Structure and conformation of chalcone photodimers and related compounds. J. Org. Chem. 1973, 38, 710–716. [Google Scholar] [CrossRef]
- Toda, F. Solid state organic chemistry: Efficient reactions, remarkable yields, and stereoselectivity. Acc. Chem. Res. 1995, 28, 480–486. [Google Scholar] [CrossRef]
- Schmidt, G.M.J. Photodimerization in the Solid State. Pure Appl. Chem. 1971, 27, 647–678. [Google Scholar] [CrossRef]
- Bart, J.C.J.; Schmidt, G.M.J. Topochemistry. Part XXXIII. The Solid-State Photochemistry of Some Anthracene Derivatives. Isr. J. Chem. 1971, 9, 429–448. [Google Scholar] [CrossRef]
- Venugopalan, P.; Rao, T.B.; Vengatesan, K. Studies in crystal engineering. Photochemical and crystallographic investigations of bromocoumarins and (±)-7-(p-bromobenzylidene)piperitone. J. Chem. Soc. Perkin Trans. 2 1991, 981–987. [Google Scholar] [CrossRef]
- Shu, Y.; Ye, K.; Yue, Y.; Sun, J.; Wang, H.; Zhong, J.; Yang, X.; Gao, H.; Lu, R. Fluorine as a robust balancer for tuning the reactivity of topo-photoreactions of chalcones crystals. Cryst. Eng. Comm. 2021, 23, 5856–5868. [Google Scholar] [CrossRef]
- Cesarin-Sobrinho, D.; Netto-Ferreira, J.C. Photochemistry of Fluorinated Chalcones in the Solid State. Quim. Nova 2002, 25, 62–68. [Google Scholar] [CrossRef]
- Lei, T.; Zhou, C.; Huang, M.-Y.; Zhao, L.-M.; Yang, B.; Ye, C.; Xiao, H.; Meng, Q.-Y.; Ramamurthy, V.; Tung, C.-H.; et al. General and Efficient Intermolecular [2+2] of Chalcones and Cinnamic Acid Derivatives in Solution through Visible Light Catalysis. Angew. Chem. Int. Ed. 2017, 56, 15407–15410. [Google Scholar] [CrossRef]
- Stewart, D.; Robertson, G.W.; Morrison, I.M. Identification of Cyclobutane-type Dimers of Substituted Cinnamic Acids by Gas Chromatography/Mass Spectrometry. Rapid Commun. Mass Spectrom. 1992, 6, 46–53. [Google Scholar] [CrossRef]
- Kohler, E.P.; Chadwell, H.M. Organic Syntheses Collective; John Wiley: New York, NY, USA, 1932; Volume I, p. 78. [Google Scholar]
- Cunha, S. Simple methods of single crystal formation of organic substance for structural study by X-ray diffraction. Quim. Nova 2008, 31, 906–909. [Google Scholar] [CrossRef]
- Duisenberg, A.J.M. Indexing in single crystal diffractometry with an obstinate list of reflections. J. Appl. Cryst. 1992, 25, 92–96. [Google Scholar] [CrossRef]
- Duisenberg, A.J.M.; Hooft, R.W.W.; Schreurs, A.M.M.; Kroon, J. Accurate cells from area-detector images. J. Appl. Cryst. 2000, 33, 893–898. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for Windows-a version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Cryst. 1997, 30, 565. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).