Abstract
This article concerns the synthesis and in silico evaluation of 1-(2-chlorophenyl)-6-7-dimethoxy-3-methyl-3,4-dihydrogioquinoline (DIQ). A variety of in silico simulations were applied to assess the potential biological activity and toxicity of the compound. Based on these analyses, the target molecule DIQ was chosen for the synthesis. DIQ was synthesized from starting 2-chloro-N-(1-(3,4-dimethoxyphenyl)propan-2-yl)benzamide applied in the Bischler–Napieralski reaction. The newly obtained 3,4-dihydroisoquinoline derivative was fully analyzed and characterized. Based on the in silico calculations, the target molecule was synthesized with respect to its contractile activity, which is a permanent interest of our studies. Thus, further investigation into the possible medicinal applications of this compound is warranted in the future.
1. Introduction
The chemistry of natural and synthetic isoquinolines is a constantly developing field of research. Their interest is determined by the wide distribution of isoquinoline substances in nature and their significant role as molecules with biological activity and compounds with practical applications in medicine [1,2].
The spectrum of biological activity of isoquinolines, substituted in different positions, depends on the nature and position of their substituents. In this respect, studies on dihydroisoquinoline synthesis containing different substitutes at the 1st, 2nd, or 4th positions have been performed [3,4]. Among the known dihydroisoquinolines, substances with cardiovascular activity, such as sympathomimetics, anticonvulsants, anticoagulants, analgesics, anti-inflammatory agents [5,6,7], a number of alkaloids, and medicinal antispasmodics exist [8]. The presence of the isoquinoline ring is preferred as a structural unit for drug design and plays an important role in the development of effective medicinal preparations for preclinical and clinical applications [9,10].
The wide range of biological activities associated with isoquinolines established our interest in finding a new approach to obtaining novel derivatives and establishing a correlation between their structure and activity. In silico simulation with the PASS online program predicts muscle contractile activity (CA) for the target molecule DIQ (1-(2-chlorophenyl)-6-7-dimethoxy-3-methyl-3,4-dihydrogioquinoline). The synthesis of a new molecule from an isoquinoline class, containing a phenyl substituent with a chlorine atom attached to a 2-phenylethylamine or substituted 2-phenylethylamines, is extremely interesting in view of what properties the newly obtained molecule would inherit from both fragments. A few representatives of the approved and well-known medicines used in medicinal practice containing chlorine atoms are chloramphenicol, chloroquine, chlorambucil, etc., used for their antibacterial, antibiotic, anticancer, and antimalarial properties, respectively [11]. Our interest was also driven by the established CA and cognitive function effects of previously described 2-chloro-N-(1-(3,4-dimethoxyphenyl)propan-2-yl)-2-phenylacetamide, a chlorine-containing N-(1-(3,4-dimethoxyphenyl)propan-2-yl) amide [12].
2. Results and Discussion
2.1. In Silico Prediction of Activity
To be effective as a drug, a molecule must reach its pharmacological target in the body, achieve an adequate concentration at the site of action, and persist in a bioactive form long enough for the expected biological events to occur. Many compounds fail as drugs because of their poor pharmacokinetic properties and limited bioavailability. A number of newly synthesized experimental molecules, on one hand, and the quantitative limitations of tissue samples, together with the need to restrict animal testing, on the other hand, prevent systematic recourse to experiments. In this context, in silico computer models constitute a valid alternative and useful addition to biological experiments [13]. In our calculations, the PASS Online Program (Prediction of Activity Spectra for Substances) was used. The program predicted CA for the compound.
In order to elucidate the ADME (absorption, distribution, metabolism, elimination) profile of the compounds, the free SwissADME web tools were used, knowing that the blood–brain barrier (BBB) and gastrointestinal absorption are pharmacokinetic characteristics that play an essential role in the drug discovery process.
Molecular weight (MW) and octanol/water partition coefficient (XLOGP3) are the drug-likeliness parameters, known as Lipinski’s rule of five [14]. According to the rule, at least two parameters from four basic pharmacokinetic properties (MW ≤ 500; XLOGP3 ≤ 5; the number of hydrogen bond donors ≤ 5; and hydrogen bond acceptors ≤ 10.6) should be fulfilled for drug candidates. Bioavailability (BA) [15] reported an optimal range of distinct properties involving lipophilicity (XLOGP3: −0.7 to +5.0), size (MW: 150 to 500 g/mole), polarity (TPSA: 20 to 130 Å2), ESOL or estimated solubility (logS: not more than 6), saturation (Fraction Csp3 or fraction of carbons in the sp3 hybridization: not less than 0.25), and flexibility (RB: no more than 9).
The ADME investigation for the DIQ shows that the compound crosses through the BBB and presents good gastrointestinal absorption, and could be considered as a drug candidate according to Lipinski’s rule of five [14], an important step in the drug discovery process. The calculated TPSA value is 30.82 Å2, indicating good intestinal absorption and BBB penetration. The low number of rotatable bonds (3) corresponds with sufficient oral bioavailability.
P-glycoproteins are pharmacokinetic proteins functioning as xenobiotic neuroprotectors, drugs, etc. [16,17]. According to our calculations, the studied compound is not predicted as a P-glycoprotein substrate. It is known that inhibition of cytochrome P450 (CYP) could lead to toxicity or lack of drug efficacy [18,19]. According to the calculations, DIQ can inhibit the CYP2C19, CYP2C9, CYP2D6, and CYP3A4 isoforms. The skin permeability factor log Kp is −4.69.
The solubility parameter is very important for the pharmaceutical applications of the drug. DIQ was predicted by SwissADME to be moderately soluble in water. The compound scored a BA of 0.55, thus demonstrating good oral bioavailability according to Lipinski’s rule [14]. Moreover, it has good synthetic accessibility (SA) scores, which is a considerable parameter during the drug discovery processes. Additionally, its predicted LD50 is 480 mg/kg.
Based on these calculations, the target molecule DIQ was synthesized as a compound with potential contractile activity and good oral bioavailability.
2.2. Synthesis of the Target Molecule
Previously, we reported a method for the synthesis of different isoquinoline precursors from starting 1-(3,4-dimethoxyphenyl)propan-2-one via the Leuckart reaction using formamide and formic acid, followed by hydrolysis with 5N H2SO4. The reaction obtained amine, which was successfully applied in a reaction with 2-chlorobenzoyl chloride to prepare amide [12].
In the present study, we focused on the incorporation of the acyl residue into the N-heterocycle using the Bischler–Napieralski reaction and further established how the substituent in the heterocyclic ring affected the CA. We found that amide 1, when refluxed for 1 h with phosphorus(V)oxychloride in 1,2-dichloroethane, yields DIQ 2 (Scheme 1).
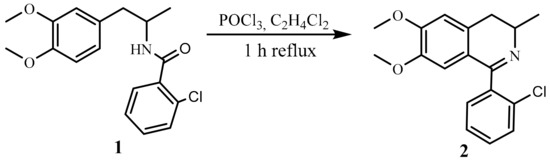
Scheme 1.
Synthesis of DIQ, 2.
This direct functionalization of dihydroisoquinolines proceeded efficiently, furnishing the desired 1-substituted 3-methyl 3,4-dihydroisoquinoline 2 (abbreviated as DIQ) in 80% yield.
The spectral data confirmed the structure of the obtained compound. In 1H-NMR spectra and 13C-NMR spectra (Supplementary Materials Figures S1 and S2), all the signals were fully consistent with the corresponding molecule. The doublet at 6.03 ppm for NH in amide 1 disappeared and two new signals for the aromatic ring in DIQ at 6.48 and 6.82 ppm appeared. The characteristic doublet for three protons (CH3) and the methoxy groups were the same as the corresponding spectra of the amide 1. In 13C-NMR of 2, the signal for the C=O group for the amide 1 at 165.8 ppm disappeared as well.
HRMS analysis verified the mass of the target compound 2 (Supplementary Materials Figure S3).
3. Materials and Methods
All reagents and chemicals were purchased from commercial sources Merck (Merck Bulgaria EAD) and Riedel-de Haën, Sofia, Bulgaria) and used as received. TLC was carried out on precoated 0.2 mm Fluka silica gel 60 plates (Merck KGaA, Darmstadt, Germany). The NMR spectral data were recorded on a Bruker Avance III HD 500 spectrometer (Bruker, Billerica, MA, USA). Chemical shifts are given in relative ppm and were referenced to tetramethylsilane (TMS) (δ = 0.00 ppm) as an internal standard; the coupling constants are indicated in Hz. The NMR spectra were recorded at room temperature (ac. 295 K). The MS analysis was performed on a Q Exactive Plus high-resolution mass spectrometer (HRMS) with a heated electrospray ionization source (HESI-II) (Thermo Fisher Scientific, Inc., Bremen, Germany) equipped with a Dionex Ultimate 3000RSLC ultrahigh-performance liquid chromatography (UHPLC) system (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
3.1. In Silico Calculations
3.1.1. Theoretical Prediction of Pharmacokinetic Parameters (ADME)
Physicochemical properties, drug-likeness, and pharmacokinetic parameters (ADME) of 2 were analyzed using SwissADME, which provides a predictive model for the pharmacokinetic profiling of a drug-like compound [18].
3.1.2. Theoretical Prediction of Toxicity
For predicting both acute and organ toxicity of the compounds, the ProToxII web tool was used, which predicts various toxicity endpoints, including acute toxicity and organ toxicities, such as hepatotoxicity, cytotoxicity, carcinogenicity, mutagenicity, immunotoxicity, and toxicity targets. Toxicity class and LD50 values were also estimated [19,20].
3.1.3. PASS Online Predictions
PASS online (Prediction of Activity Spectra for Substances), a computer-based program was used to screen the biological activity of the compound. The program predicts several thousand different biological activities based on the structural formula of a drug-like organic compound [21]. PASS has been used by many scientists for the discovery of new pharmaceutical agents in different therapeutic fields [22,23].
3.2. Synthetic Method
To a solution of 3 mmol (0.999 g) of the amide 1 in 30 mL 1,2-dichloroethane, 1 mL phosphorus(V) oxychloride was added. The reaction mixture was refluxed for 1 h at 100 °C (TLC), then poured in water and extracted with CH2Cl2 (3 × 20 mL) and consequently washed with Na2CO3 and water. The organic layer was dried using anhydrous Na2SO4, filtered on the short column filled with basic Al2O3, and then concentrated.
1-(2-chlorophenyl)-6,7-dimethoxy-3-methyl-3,4-dihydroisoquinoline (DIQ, 2): yellow liquid, 80% yield, 1H-NMR: 1.39 (d, J = 6.3, 3H, CH-CH3), 2.95 (dd, J = 13.6, 4.6, 1H, CH2), 3.24 (dd, J = 13.6, 10.8, 1H, CH2), 3.59 (s, 3H, OCH3), 3.89 (s, 3H, OCH3), 4.39–4.46 (m, 1H, CH-CH3), 6.48 (s, 1H, Ar), 6.82 (s, 1H, Ar), 7.17 (s, 1H, Ar), 7.46–7.53 (m, 3H, Ar); 13C-NMR: 147.6, 147.1, 134.0, 131.1, 129.75, 129.4, 128.68, 128.3, 128.0, 127.4, 111.3, 110.28, 55.87, 49.9, 37.6, 22.38. HRMS Electrospray ionization (ESI) m/z calcd for [M + H]+ (composition C18H19O2NCl) = 316.10988, found 316.10874 (mass error Δm = 1.14 ppm)
4. Conclusions
A series of in silico calculations were performed to predict the biological activity, ADME properties, and toxicity of a novel 3,4-dihydroisoquinoline 2. The compound was predicted to possess CA and good oral bioavailability. Based on the in silico calculations, DIQ was synthesized using the Bischler–Napieralski reaction. In the future, we plan to carry out a comparative study of the spasmolytic activity and the cognitive function between the newly synthesized isoquinoline 2 and its precursor 1. Thus, further investigation into the possible medicinal applications of this compound is warranted in the future.
Supplementary Materials
Figure S1: 1H-NMR spectrum of 2, Figure S2: 13C-NMR spectrum of 2; Figure S3: HRMS of 2.
Author Contributions
Conceptualization, S.N.; methodology, M.M. and V.G.; investigation, V.G., M.M. and I.S.; writing—original draft preparation, S.N. and M.M.; writing—review and editing, S.N. and M.M.; visualization, V.G. and S.N.; supervision, I.S. and S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This research is supported by Scientific Project No KP-06-M63/8 of the National Fund for Scientific Research in Bulgaria.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhadra, K.; Suresh, K.G. Therapeutic potential of nucleic acid-binding isoquinoline alkaloids: Binding aspects and implications for drug design. Med. Res. Rev. 2011, 31, 821–862. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Y.; Kumar, G.S. Natural isoquinoline alkaloids: Binding aspects to functional proteins, serum albumins, hemoglobin, and lysozyme. Biophys. Rev. 2015, 7, 407–420. [Google Scholar] [CrossRef]
- Ivanov, I.; Nikolova, S.; Aladjov, D.; Stefanova, I.; Zagorchev, P. Synthesis and Contractile Activity of Substituted 1,2,3,4-Tetrahydroisoquinolines. Molecules 2011, 16, 7019–7042. [Google Scholar] [CrossRef]
- Kmieciak, A.; Ćwiklińska, M.; Jeżak, K.; Shili, A.; Krzemiński, M.P. Searching for New Biologically Active Compounds Derived from Isoquinoline Alkaloids. Chem. Proc. 2021, 3, 97. [Google Scholar] [CrossRef]
- Qing, Z.-X.; Yang, P.; Tang, Q.; Cheng, P.; Liu, X.-B.; Zheng, Y.J.; Liu, Y.-S.; Zeng, J.-G. Isoquinoline alkaloids and their antiviral, antibacterial, and antifungal activities and structure-activity relationship. Curr. Org. Chem. 2017, 21, 1. [Google Scholar] [CrossRef]
- Qian, J.-Q. Cardiovascular pharmacological effects of bisbenzylisoquinoline alkaloid derivatives. Acta Pharmacol. Sin. 2002, 23, 1086–1092. [Google Scholar]
- Kukula-Koch, W.; Widelski, J. Alkaloids. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 163–198. [Google Scholar] [CrossRef]
- Ahmed, W.; Huang, Z.-H.; Cui, Z.-N.; Tang, R.-Y. Design and synthesis of unique thiazoloisoquinolinium thiolates and derivatives. Chin. Chem. Lett. 2021, 32, 3211–3214. [Google Scholar] [CrossRef]
- Bentley, K.W. β-Phenylethylamines and the isoquinoline alkaloids. Nat. Prod. Rep. 2001, 18, 148–170. [Google Scholar] [CrossRef]
- Luo, C.; Ampomah-Wireko, M.; Wang, H.; Wu, C.; Wang, Q.; Zhang, H.; Cao, Y. Isoquinolines: Important Cores in Many Marketed and Clinical Drugs. Anticancer Agents Med. Chem. 2021, 21, 811–824. [Google Scholar] [CrossRef]
- Shaik, A.B.; Bhandare, R.R.; Nissankararao, S.; Edis, Z.; Tangirala, N.R.; Shahanaaz, S.; Rahman, M.M. Design, Facile Synthesis and Characterization of Dichloro Substituted Chalcones and Dihydropyrazole Derivatives for Their Antifungal, Antitubercular and Antiproliferative Activities. Molecules 2020, 25, 3188. [Google Scholar] [CrossRef]
- Milusheva, M.; Gledacheva, V.; Batmazyan, M.; Nikolova, S.; Stefanova, I.; Dimitrova, D.; Saracheva, K.; Tomov, D.; Chaova-Gizdakova, V. Ex Vivo and In Vivo Study of Some Isoquinoline Precursors. Sci. Pharm. 2022, 90, 37. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. Swiss ADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Martin, Y.C. A bioavailability score. J. Med. Chem. 2005, 48, 3164–3170. [Google Scholar] [CrossRef]
- Pathan, N.; Shende, P. Tailoring of P-glycoprotein for effective transportation of actives across blood-brain-barrier. J. Control. Release 2021, 335, 398–407. [Google Scholar] [CrossRef]
- Testa, B.; Kraemer, S.D. The biochemistry of drug metabolism—An introduction: Part 4. Reactions of conjugation and their enzymes. Chem. Biodivers. 2008, 5, 2171–2336. [Google Scholar] [CrossRef]
- Isyaku, Y.; Uzairu, A.; Uba, S. Computational studies of a series of 2-substituted phenyl-2-oxo-, 2-hydroxyl- and 2-acylloxyethylsulfonamides as potent anti-fungal agents. Heliyon 2020, 6, e03724. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Mazumder, K.; Hossain, E.; Aktar, A.; Mohiuddin, M.; Sarkar, K.K.; Biswas, B.; Aziz, A.; Abid, A.; Fukase, K. In Silico Analysis and Experimental Evaluation of Ester Prodrugs of Ketoprofen for Oral Delivery: With a View to Reduce Toxicity. Processes 2021, 9, 2221. [Google Scholar] [CrossRef]
- Anzali, S.; Barnickel, G.; Cezanne, B.; Krug, M.; Filimonov, D.; Poroikov, V. Discriminating between Drugs and Nondrugs by Prediction of Activity Spectra for Substances (PASS). J. Med. Chem. 2001, 44, 2432–2437. [Google Scholar] [CrossRef]
- Mathew, B.; Suresh, J.; Anbazhagan, S. Synthesis and PASS-assisted in silico approach of some novel 2-substituted ben-zim-idazole bearing a pyrimidine-2,4,6 (trione) system as mucomembranous protector. J. Pharm. Bioallied Sci 2013, 5, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Ekins, S.; Olechno, J.; Williams, A.J. Dispensing Processes Impact Apparent Biological Activity as Determined by Computational and Statistical Analyses. PLoS ONE 2013, 8, e62325. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).