Abstract
Berberine is a phytogenic isoquinoline alkaloid which demonstrates several pharmacological effects, including a hypoglycemic effect. Its medical use is limited by its very low bioavailability. Synthesizing new berberine derivatives might help in overcoming this problem. In this work, we report on the synthesis and biological evaluation of a novel berberine 9-O-derivative. At an oral dose of 25 mg/kg, the compound demonstrated hypoglycemic activity in an oral glucose tolerance test performed using C57BL/6 mice.
1. Introduction
Berberine 1a (Figure 1) is an isoquinoline alkaloid found in several plants such as Berberis aristata, Coptis chinensis, etc. This compound has found applications in medicine as an antibacterial, hypolipidemic and hypoglycemic agent. However, its use is limited by a very low bioavailability (the clinical dosage of berberine is 380 mg/kg) [1]. Currently, one of the promising areas of research is the synthesis of new, berberine-based derivatives as potential hypoglycemic agents. It was previously noted that the introduction of alkyl and acyl substituents at position 9 of the berberine backbone contributes to an increase in hypoglycemic activity. However, the therapeutic doses of these derivatives, 1b and 1c (Figure 1), are still high and vary in the range of 100–200 mg/kg [2,3,4]. The lowest effective hypoglycemic doses (30 mg/kg or less) of berberine derivatives found in the literature were described for the series of 9-N-substituted derivatives 2 (Figure 1) [5,6,7].
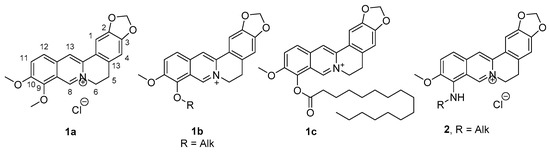
Figure 1.
Structures of berberine and its derivatives with hypoglycemic activity.
In this work, we have synthesized a new 9-O-derivative of berberine with a substituent containing a fragment of a tertiary aromatic amide of acetic acid. The resulting compound was tested for hypoglycemic activity in an oral glucose tolerance test (OGTT) in mice.
2. Results
2.1. Chemistry
A new 9-O-derivative of berberine with a substituent containing a fragment of a tertiary aromatic acetic amide was synthesized according to Scheme 1.
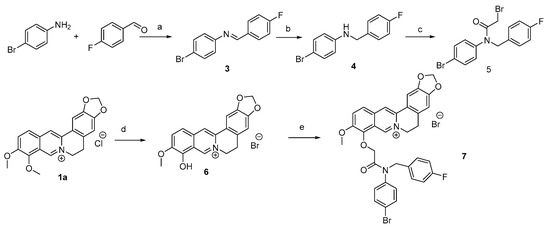
Scheme 1.
Synthesis of compound 7. Reagents and conditions: (a) MeOH, 83%; (b) NaBH3CN, MeOH, 53%; (c) BrC(O)CH2Br, CH2Cl2, NEt3, 53%; (d) 180–200 °C, 20–30 mm Hg; HBr, 89%; and (e) bromide 5, AcCN, NEt3, 75%.
The first stage was the synthesis of imine 3 from 4-fluorobenzaldehyde and 4-bromoaniline. The second stage was the reduction of imine 3 to the corresponding secondary amine 4 by the action of cyanoborohydride. The third stage was an interaction with bromoacetic acid bromide, obtained by the action of PBr3 and Br2 on acetic acid, according to the Hell–Volgard–Zelinsky reaction. Compound 5 was purified by column chromatography.
Thermal demethylation of berberine 1 was carried out under reduced pressure at 180–200 °C. This was followed by treatment with hydrobromic acid, which led to the formation of berberrubine hydrobromide 6 in an 89% yield. The reaction of berberrubine hydrobromide 9 with bromoacetic acid amide 5 in acetonitrile in the presence of triethylamine gave the target 9-O-substituted berberine derivative 7 in a 75% yield.
2.2. Biology
In order to assess the hypoglycemic activity of the synthesized compound 7, an OGTT was performed using C57BL/6 mice. The resulting data showed that compound 7 promoted a statistically significant reduction in blood glucose levels compared to the control group 60, 90, and 120 min after the glucose administration (Figure 2). Additionally, despite the fact that its activity was initially not as pronounced as that of the reference drug vildagliptin (VLD), the intensities of their hypoglycemic effects were almost identical in the second half of the experiment. The area under the glycemic curve (AUC) was also significantly lower in the mice treated by 7 compared to the control group and higher than in the VLD group, proving the above-mentioned observations (Figure 3). At a dose of 30 mg/kg, berberine itself, which was studied in our group earlier (not published data) in a similar experiment, did not demonstrate any hypoglycemic action, and its glycemic curve was similar to the control group. In summary, it can be concluded that, at an oral dose of 25 mg/kg, compound 7 possesses a mild hypoglycemic activity. Since the mechanism of the hypoglycemic action of berberine itself may involve a wide range of targets [1,3,6,8], it can be assumed that compound 7 also exerts its action through one or more similar mechanisms of action. However, further studies are needed for a more precise statement.
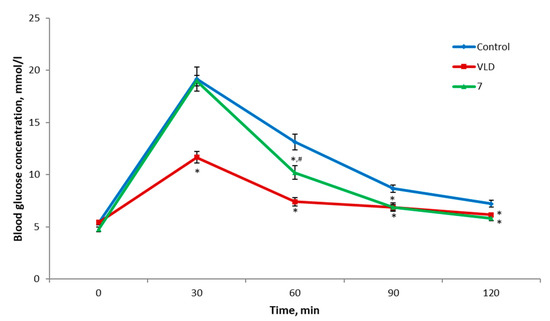
Figure 2.
Results of the OGTT (mice n = 6 in each group). * p < 0.05 compared to the control, # p < 0.05 as compared to the VLD 10 mg/kg. VLD—vildagliptin.
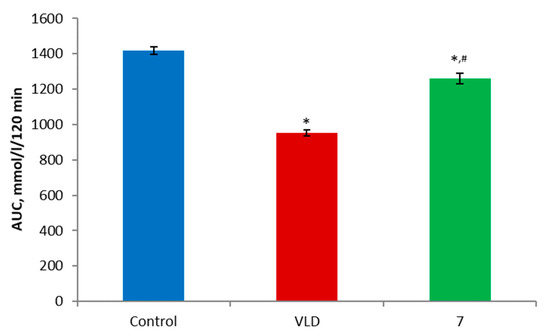
Figure 3.
Area under the glycemic curve calculated according to the OGTT data. * p < 0.05 as compared to the control, # p < 0.05 as compared to the VLD. VLD—vildagliptin.
3. Materials and Methods
Synthetic starting materials and reagents were acquired from Acros Organics. Berberine chloride hydrate was purchased from TCI Co. (Tokyo, Japan) and was used after drying in an oven at 95 °C for 5 h.
1H and 13C NMR spectra were acquired using a Bruker spectrometer AV-400 (Bruker Corporation, Bremen, Germany) at 400.13 MHz (1H) and 100.61 MHz (13C). Spectra were recorded in deuterated chloroform (CDCl3); residual CDCl3 was used as a standard [δ(CDCl3) 7.24, δ(CDCl3) 77.0 ppm] for measuring chemical shifts, δ, in parts per million (ppm), and J was measured in Hertz. The infrared spectra (IR) were measured on a Vector 22 FTIR spectrometer in potassium bromide (KBr) pellets. The structures of the product and intermediate compounds were determined by means of 1H and 13C NMR spectra (Figures S1–S3). For the column chromatography, silica gel (60–200 mesh, Macherey-Nagel) was used.
3.1. Synthesis
Berberrubine hydrobromide 6 was synthesized according to the procedure described in [9].
Additionally, 2-bromoacetyl bromide was synthesized according the procedure described in [10]. Compound 3 was synthesized according to the procedure described in [11].
Synthesis of 2-Bromoacetic acid N-(4a-fluorobenzyl)-N-(4a-bromophenyl)amide 5 was achieved.
Sodium cyanoborohydride (1.2 mmol) was added to a solution of 4.82 mmol of imine 3 in 16 mL of methanol with cooling (0 °C) and stirring. The mixture was left to stir at room temperature for 2.5 h. The reaction mixture was evaporated. Chloroform (2 mL) was added to the evaporated residue. A supernatant solution in chloroform was separated by column chromatography (eluent chloroform). The yield of compound 4 was 53%.
4-Bromo-N-(4-fluorobenzyl)aniline 4.
NMR 1H (CDCl3): 4.05 (1H, s, NH), 4.25 (2H, s, NCH2), 6.47 (2H, d, J = 8.7 Hz,), 7.01 (2H, t, J = 8.6 Hz,), 7.23 (2H, d, J = 8.9 Hz,), 7.29 (2H, dd, J1 = 5.5 Hz, J2 = 8.3 Hz).
NMR 13C (CDCl3): 47.40, 109.18, 114.31, 115.40 (d, J = 21.3 Hz), 128.79 (d, J = 8.0 Hz), 131.83, 134.39 (d, J = 3.3 Hz), 146.7, 161.97 (d, J = 245.5 Hz).
An amount of 3.43 mmol of triethylamine was added to a solution of 3.43 mmol of compound 4 in 6 mL of methylene chloride. While cooling (0 °C) and stirring, 3.43 mmol of bromoacetic acid, bromide 2 dissolved in 3 mL of methylene chloride, was added dropwise. The reaction was left to stir at room temperature for 2.5 h. At the end of the reaction, 10 mL of ethyl acetate was added. The formed precipitate was filtered off. The mother liquor was evaporated, and compound 5 was isolated by chromatography (eluent methylene chloride). The yield was 53%. Mp 72.4–75.0 °C. IR (KBr), ν/см−1: 1670, 1508, 1389, 1308, 1228, 1008, 835, 698. Anal. Calcd. for C15H12Br2FNO (401.07): C, 44.92; H, 3.02; N, 3.49; F, 4.74; Br, 39.85 %. Br, 13.65%. Found: C, 39.45; H, 3.45; N, 3.62; F, 4.79; Br, 39.98%.
2-Bromoacetic acid N-(4-fluorobenzyl)-N-(4-bromophenyl)amide 5.
NMR 1H (CDCl3): 3.61 (2H, s, CH2Br), 4.79 (2H, s, NCH2), 6.93 (4H, m), 7.12 (2H, m), 7.47 (2H, d, J = 8.5 Hz).
NMR 13C (CDCl3): 26.37, 52.40, 115.12 (d, J = 21.3 Hz), 122.45, 129.51, 130.35 (d, J = 8.0 Hz), 131.69 (d, J = 3.3 Hz), 132.73, 139.47, 161.94 (d, J = 246.6 Hz), 165.81.
Synthesis of compound 7.
First, 3.5 mmol of triethylamine was added to a suspension of 1 mmol of berberrubine hydrobromide 6 in acetonitrile (10 mL), followed by a solution of 1 mmol amide 5 dissolved in acetonitrile. The mixture was refluxed for 4 h. The reaction mixture was cooled, and the precipitate (compound 7) was filtered off and recrystallized from acetonitrile. The yield was 75%.
9-O-(N-(4-bromophenyl)-N-(4-fluorobenzyl)-acetamide-2,3-methylenedioxy-10-methoxyprotoberberine bromide 7.
Mp 244.2 °C (decomposition). IR (KBr), ν/см−1: 3554, 3417, 3031, 2942, 1674, 1508, 1394, 1275, 1230, 1101, 829. Anal. Calcd. for C34H27Br2FN2O (722.40): C, 53.47; H, 4.07; N, 3.55; Br, 21.82; F, 2.89%. Found:C, 56.53; H, 3.77; N, 3.88; Br, 22.12; F, 2.63%.
NMR 1H (DMSO-d6): 3.22 (2H, t, J = 6.0 Hz, H-5), 3.94 (3H, s, OCH3), 4.84 (2H, s, OCH2CO), 4.93 (4H, m), 6.17 (2H, s, OCH2O), 7.10 (3H, m), 7.19 (4H, m), 7.63 (2H, d, J = 8.3 Hz), 7.80 (1H, s), 7.95 (1H, d, J = 9.1 Hz), 8.15 (1H, d, J = 9.1 Hz), 8.93 (1H, s), 9.96 (1H, s).
NMR 13C (DMSO-d6): 26.73, 51.49, 55.86, 57.45, 70.12, 102.41, 105.79), 108.79, 115.57 (d, J = 21.3 Hz), 120.31, 120.69, 121.60, 121.91, 123.56, 126.91, 130.54 (d, J = 6.4 Hz), 130.97, 133.09 (d, J = 3.3 Hz), 133.14, 133.32, 137.80, 139.28, 142.12, 146.20, 148.02, 149.31, 150.20, 161.75 (d, J = 243.2 Hz), 167.62.
3.2. Animals
In the present study, male C57BL/6 mice, weighing 22–25 g, from the SPF vivarium of the Institute of Cytology and Genetics SB RAS were used. The animals were housed in polycarbonate cages in the vivarium room, in which the humidity, temperature, and the 12/12 h light-and-dark cycle were controlled. The mice had ad libitum access to water and pelleted feed. All manipulations with animals were conducted in strict accordance with the laws of the Russian Federation, the decree of the Ministry of Health of the Russian Federation no. 199n of 4 January 2016, and Directive 2010/63/EU of the European Parliament and of the Council of the European Union of 22 September 2010 on the protection of animals used for scientific purposes.
3.3. OGTT
The hypoglycemic effect of the tested compound was studied using an oral glucose tolerance test on C57BL/6 mice. There were six animals in each experimental group. The studied substance 7 (25 mg/kg) was dissolved in water with a drop of Tween 80. Vildagliptin (Galvus, Novartis Farmaceutica SA, Barcelona, Spain) was used as a reference drug at a dose of 10 mg/kg. The control group was treated only with the vehicle (water with a drop of Tween 80). All substances were administered by oral gavage after a 12 h fast. Then, 30 min later, all animals received glucose at a dose of 2.5 g/kg by the same method. Blood was collected by a tail snip before the experiment (0 time point) and then every 30 min for 2 h after drug administration. The glucose concentration in the collected blood was measured using a ONE TOUCH Select glucometer (LIFESCAN Inc., Milpitas, CA, USA). The AUC was calculated for each group using Tai’s model [12].
3.4. Statistical Analysis
A statistical analysis was performed using the Mann–Whitney U test. Data are shown as mean ± SEM. Data with p < 0.05 were considered statistically significant.
4. Conclusions
Based on previously published data on the hypoglycemic activity of berberine derivatives, we reported the synthesis of a novel berberine 9-O derivative with a substituent containing a tertiary aromatic acetic amide moiety. The biological assessment showed that, at an oral dose of 25 mg/kg, compound 7 possesses a mild hypoglycemic activity.
Supplementary Materials
The following supporting information can be downloaded, Figures S1–S3. 1H, 13C NMR, IR spectra of compound 7.
Author Contributions
Conceptualization, O.A.L. and M.V.K.; methodology, O.A.L. and M.V.K.; investigation, E.D.G., N.V. and S.A.B.; data curation, O.A.L. and M.V.K.; writing—original draft preparation, O.A.L., M.V.K. and S.A.B.; writing—review and editing, O.A.L. and M.V.K.; supervision, N.F.S. and T.G.T. All authors have read and agreed to the published version of the manuscript.
Funding
The work was done within state assignments to N.N. Vorozhtsov Novosibirsk Institute of Organic Chemistry SB RAS (projects No. 1021051402785-4-1.4.1 and No 1021051703312-0-1.4.1).
Institutional Review Board Statement
The protocol of the animal experiment was approved by the Ethics Committee of N.N. Vorozhtsov Institute of Organic Chemistry SB RAS (protocol no. p-10-05.2021-14, 16.05.2021).
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge the Multi-Access Chemical Research Center SB RAS for spectral and analytical measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xia, X.; Yan, J.; Shen, Y.; Tang, K.; Yin, J.; Zhang, Y.; Yang, D.; Liang, H.; Ye, J.; Weng, J. Berberine Improves Glucose Metabolism in Diabetic Rats by Inhibition of Hepatic Gluconeogenesis. PLoS ONE 2011, 6, e16556. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; He, L.; Yang, G. Synthesis and antihyperglycemic evaluation of various protoberberine derivatives. Bioorg. Med. Chem. Lett. 2006, 16, 1380–1383. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Huang, R.; Li, Q.; Liu, Y.; Li, Y.; Chen, H.; Ai, G.; Xie, J.; Zeng, H.; Chen, J.; et al. Oxyberberine, an absorbed metabolite of berberine, possess superior hypoglycemic effect via regulating the PI3K/Akt and Nrf2 signaling pathways. Biomed. Pharmacother. 2021, 137, 111312. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Wang, Y.-X.; Li, Y.-H.; Song, D.-Q.; Kong, W.-J.; Jiang, J.-D. Structure-activity relationship of berberine derivatives for their glucose-lowering activities. Int. J. Clin. Exp. Med. 2017, 10, 5054–5060. [Google Scholar]
- Khvostov, M.V.; Gladkova, E.D.; Borisov, S.A.; Fedotova, M.S.; Zhukova, N.A.; Marenina, M.K.; Meshkova, Y.V.; Valutsa, N.; Luzina, O.A.; Tolstikova, T.G.; et al. 9-N-n-alkyl Berberine Derivatives: Hypoglycemic Activity Evaluation. Pharmaceutics 2023, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Khvostov, M.V.; Gladkova, E.D.; Borisov, S.A.; Fedotova, M.S.; Zhukova, N.A.; Marenina, M.K.; Meshkova, Y.V.; Luzina, O.A.; Tolstikova, T.G.; Salakhutdinov, N.F. Study of Hypoglycemic Activity of Novel 9-N-alkyltetrahydroberberine Derivatives. Int. J. Mol. Sci. 2022, 23, 14186. [Google Scholar] [CrossRef] [PubMed]
- Khvostov, M.V.; Gladkova, E.D.; Borisov, S.A.; Zhukova, N.A.; Marenina, M.K.; Meshkova, Y.V.; Luzina, O.A.; Tolstikova, T.G.; Salakhutdinov, N.F. Discovery of the First in Class 9-N-Berberine Derivative as Hypoglycemic Agent with Extra-Strong Action. Pharmaceutics 2021, 13, 2138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, H.; Yang, E.; Li, J.; Dong, L. Berberine Decreases Intestinal GLUT2 Translocation and Reduces Intestinal Glucose Absorption in Mice. Int. J. Mol. Sci. 2022, 23, 327. [Google Scholar] [CrossRef] [PubMed]
- Nechepurenko, I.V.; Shirokova, E.D.; Khvostov, M.V.; Frolova, T.S.; Sinitsyna, O.I.; Maksimov, A.M.; Bredikhin, R.A.; Komarova, N.I.; Fadeev, D.S.; Luzina, O.A.; et al. Synthesis, hypolipidemic and antifungal activity of tetrahydroberberrubine sulfonates. Russ. Chem. Bull. 2019, 68, 1052–1060. [Google Scholar] [CrossRef]
- Ward, C.F. The bromination of acids in the α-position. J. Chem. Soc. 1922, 121, 1161–1165. [Google Scholar] [CrossRef]
- Ragaini, F.; Penoni, A.; Gallo, E.; Tollari, S.; Gotti, C.L.; Lapadula, M.; Mangioni, E.; Cenini, S. Amination of Benzylic C-H Bonds by Arylazides Catalyzed by CoII–Porphyrin Complexes: A Synthetic and Mechanistic Study. Chem. Eur. J. 2003, 9, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Tai, M.M. A Mathematical Model for the Determination of Total Area under Glucose Tolerance and Other Metabolic Curves. Diabetes Care 1994, 17, 152–154. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).