4,4′-Difluoro-[3,3′-bi(1,2,5-oxadiazole)] 2,2′-Dioxide
Abstract
1. Introduction
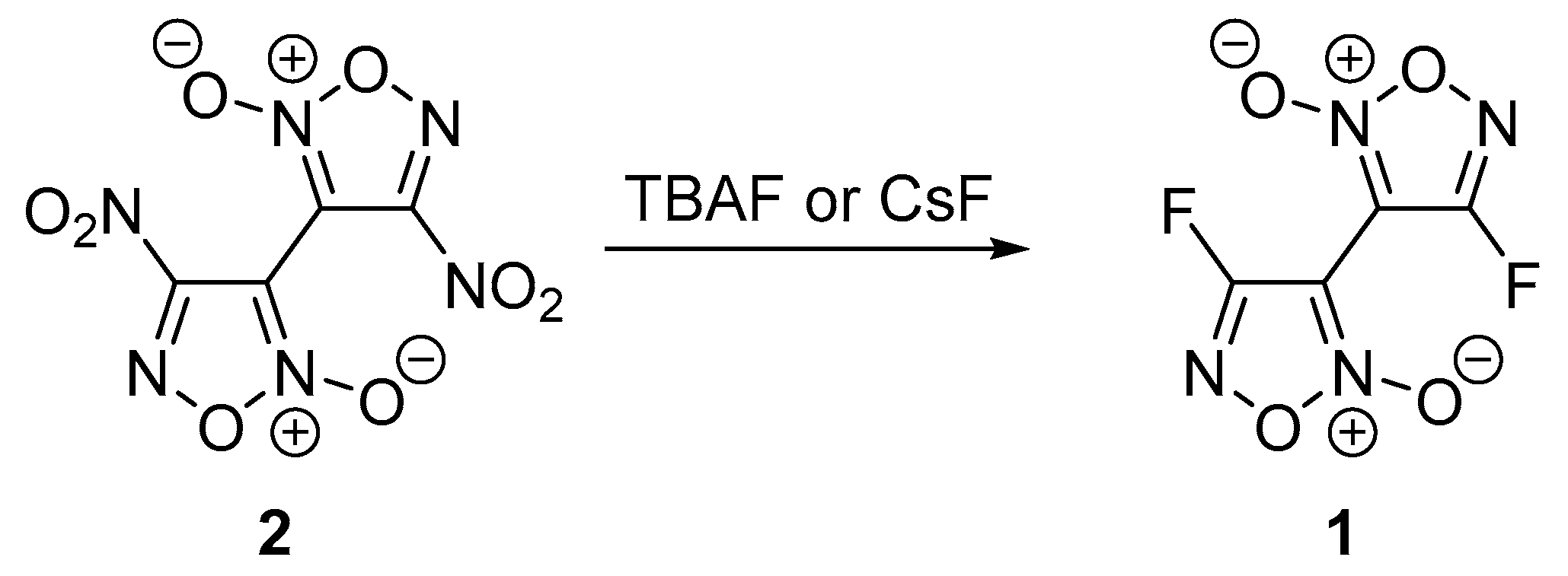
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gasco, A.; Fruttero, R.; Sorba, G.; Di Stilo, A.; Calvino, R. NO Donors: Focus on Furoxan Derivatives. Pure Appl. Chem. 2004, 76, 973–981. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Makhova, N.N. Molecular Hybridization Tools in the Development of Furoxan-Based NO-Donor Prodrugs. ChemMedChem 2017, 12, 622–638. [Google Scholar] [CrossRef]
- Makhova, N.N.; Fershtat, L.L. 5.05-1,2,5-Oxadiazoles. In Comprehensive Heterocyclic Chemistry IV; Elsevier: Amsterdam, The Netherlands, 2022; pp. 190–251. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Zhilin, E.S. Recent Advances in the Synthesis and Biomedical Applications of Heterocyclic NO-Donors. Molecules 2021, 26, 5705. [Google Scholar] [CrossRef]
- Hwang, K.-J.; Kim, S.K.; Shim, S.C. Photochemical Generation of Nitric Oxide from 3,4-Bis-2′-Chlorophenylfuroxan. Chem. Lett. 1998, 27, 859. [Google Scholar] [CrossRef]
- Auricchio, S.; Selva, A.; Truscello, A.M. New Reactions of Furoxans: Formation of Alkynes and Cyclobutaphenanthrenes. Tetrahedron 1997, 53, 17407–17416. [Google Scholar] [CrossRef]
- Seymour, C.P.; Tohda, R.; Tsubaki, M.; Hayashi, M.; Matsubara, R. Photosensitization of Fluorofuroxans and Its Application to the Development of Visible Light-Triggered Nitric Oxide Donor. J. Org. Chem. 2017, 82, 9647–9654. [Google Scholar] [CrossRef]
- Ando, A.; Matsubara, R.; Takazawa, S.; Shimada, T.; Hayashi, M. Fluorofuroxans: Synthesis and Application as Photoinduced Nitric Oxide Donors. Asian J. Org. Chem. 2016, 5, 886–890. [Google Scholar] [CrossRef]
- Larin, A.A.; Shaferov, A.V.; Epishina, M.A.; Melnikov, I.N.; Muravyev, N.V.; Ananyev, I.V.; Fershtat, L.L.; Makhova, N.N. Pushing the Energy-Sensitivity Balance with High-Performance Bifuroxans. ACS Appl. Energy Mater. 2020, 3, 7764–7771. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obruchnikova, N.V.; Rakitin, O.A. 4,4′-Difluoro-[3,3′-bi(1,2,5-oxadiazole)] 2,2′-Dioxide. Molbank 2023, 2023, M1596. https://doi.org/10.3390/M1596
Obruchnikova NV, Rakitin OA. 4,4′-Difluoro-[3,3′-bi(1,2,5-oxadiazole)] 2,2′-Dioxide. Molbank. 2023; 2023(1):M1596. https://doi.org/10.3390/M1596
Chicago/Turabian StyleObruchnikova, Natalia V., and Oleg A. Rakitin. 2023. "4,4′-Difluoro-[3,3′-bi(1,2,5-oxadiazole)] 2,2′-Dioxide" Molbank 2023, no. 1: M1596. https://doi.org/10.3390/M1596
APA StyleObruchnikova, N. V., & Rakitin, O. A. (2023). 4,4′-Difluoro-[3,3′-bi(1,2,5-oxadiazole)] 2,2′-Dioxide. Molbank, 2023(1), M1596. https://doi.org/10.3390/M1596






