Abstract
The protocol, 3-methyldec-1-yn-3-ol (1a) was chosen to perform the dimerization process. The optimal conditions for synthesis of 8,13-dimethylicosa-9,11-diyne-8,13 (2a) with high efficiency when using copper-catalyzed, N,N,N′N′-tetramethylethylenediamine as a ligand and CCl4 and methanol solvents in atmospheric pressure were determined. The structure of the obtained compound was proved by IR, 1H-NMR and 13C-NMR spectroscopy.
1. Introduction
Recently, the attention of chemists, technologists and pharmacologists has focused on the elaboration and implementation of new drugs with effective pharmacological effects [1]. In this regard, diacetylene diols and their derivatives were attributed to biologically active organic compounds with pharmacological properties, such as anti-cancer [2,3], anti-bacterial [4], anti-HIV [5], anti-fungal [6] and anti-inflammatory [7,8,9,10]. Moreover, when the pharmacology of mono and diacetylene diol derivatives, including panaxynol, panoxydol, panaxydolcglorohydrin, 1,8-heptadecadiene-4,6-diyne-3,10-diol, panaxytriol and dihydropanaxacol, was studied, some of them were used for the treatment of alopecia (a disease with hair loss) due to their hair growth properties [11]. It was found that heptadeca-8,16-diene-4,6-diyne-3,10-diol extracted from the plant Aralia dumetorum possesses inhibitory properties against harmful toxins [12].
Glaser first synthesized 1,4-diphenylbuta-1,3-diyne from phenylacetylene through the dimerization reaction in the presence of copper (I) chloride and oxygen [13]. J.S. Yadav and his team synthesized 1,4-diphenyl-1,3-butadiene with 95% yield from phenylacetylene based on oxidative homocoupling–dimerization reactions 1-butyl-3-methylimidazolium hexafluorophosphate (bmim-PF6) and 1-butyl-3-methylimidazolium tetrafluoroborate (bmim-BF4) in the presence of copper (I) chloride (0.2 mol%), TMEDA (0.2 mol%) in oxygen atmosphere [14]. Yun Lia, Reza Fathi and others succeeded in synthesizing symmetrical bis-benzo[b]furan-linked 1,3-diyne through homocoupling of three bonds using the new catalytic system AgOTs−CuCl2−TMEDA. It was also observed for the first time that the reaction rate of the oxidative acetylenic homocoupling process based on activated Ag (I) cation with triple bond and Cu (II) cation was very high, the product was obtained with a yield of 70–90% and the reaction mechanism was proposed [15]. Several derivatives of 1,3-diynes, such as 1,4-dip-tolylbuta-1,3-diyne, 1,4-diphenylbuta-1,3-diyne, 1,4-bis(4-trifluoromethyl)phenyl)buta-1,3-diyne, 1,4-bis(4-(trifluoromethyl) phenyl)buta-1,3-diyne, 1,4-bis(4-methoxyphenyl)buta-1,3-diyne, 1-(cyclohex-3-enyl)-4-cyclohexenylbuta-1,3-diyne, 1,1′-buta-1,3-diyne-1,4-diyldicyclohexanol, hexadeca-7,9-diyne and dodeca-5,7-diynedinitrile (produced with 78-87% yields) were synthesized when the oxidative homocoupling reaction was carried out in the presence of catalyst Pd(OAc)2 or PdCl2(PPh3)2 and 1-(2-pyridylethynyl)-2-(2-thienylethynyl)benzene as a ligand and solvent NEt3 [16]. Diacetylene diols such as octa-3,5-diyne-1,8-diol and 2,7-dimethyl-3,5-octadiyne-2,7-diol were efficiently formed when the homocoupling reaction was carried out from terminal acetylene alcohols using the Cu(OAc)2/MgAl–LDH/TMEDA/CH3CN catalytic system [17]. Piperidine, Bu3N, Bu2NH and several solvents (toluene, 1,4-dioxane, Cl2CHCHCl2, DMSO and DMF) were studied as various additives to increase the catalytic activity to obtain with high yield in the dimerization reaction of heptin-1 in the presence of the CuCl catalyst. Tetradeca-6,8-diyne was synthesized with high yield when the process was carried out at 60 °C in a 2:10 mol ratio of toluene solvent and CuCl:piperidine, respectively [18].
2. Results and Discussion
In this work, the dimerization reaction of terminal acetylene alcohol 3-methyldec-1-yn-3-ol (1a) was carried out for the first time. Alternative conditions for the synthesis of 8,13-dimethylicosa-9,11-diyne-8,13-diol (2a) containing two triple bonds and two hydroxy (-OH) groups were analyzed. Synthesis of 2a was carried out through the dimerization reaction of terminal acetylene alcohol as 1a in the presence of CuCl/TMEDA/CCl4/MeOH catalytic system. The reaction scheme is given below (Scheme 1).
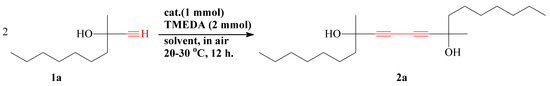
Scheme 1.
Synthesis of 2a.
The dimerization process was studied at of 20–30 °C with CuCl, CuJ and CuBr (1.0 mmol) catalysts in TMEDA (N,N,N′N′-tetramethylethylenediamine) (2.0 mmol), CCl4 in various solvents—MeCN, THF, CH2OHCH2OH, iPrOH, ethanol and methanol (Table 1).

Table 1.
Optimization of catalyst and solvent effect.
When the process was carried out for 8 h in the catalytic system CuCl/TMEDA/MeCN/CCl4, a very small amount of the product was formed on entry. 2a was obtained with low yield of 42 and 27%, respectively, when the process was carried out in THF or ethylene glycol with CCl4 for 10 h, and this increase in the time did not affect the yield (2–3 entries). The effects of CCl4 solvents with iPrOH or EtOH were studied; 2a gave moderate yields (4–5 entries). In MeOH/CCl4 for 12 h, 2a gave the best yield of 82% (six entries) compared to the others. However, in MeOH/CCl4 without addition of this catalytic system, a very small amount of 2a was obtained (seven entries). Additionally, when CuJ or CuBr (1 mmol) were used instead of CuCl, a decrease in the yield of 2a was observed (8–9 entries). Based on the research, it was concluded that CuCl has high catalytic activity compared to CuJ and CuBr and based on the oxidative homocoupling reaction of 1a, the most alternative conditions for the synthesis of 2a were determined to be the process: the CuCl/TMEDA/CCl4/MeOH catalytic system and time 12 h.
The structure of 2a obtained by the dimerization reaction of acetylene alcohols was confirmed by spectroscopic methods. (1H, 13C-NMR and IR; see also Supplementary Materials). In particular, the chemical shift region of 2.41 ppm in the 1H-NMR spectrum of the starting product 1a (Figure S1), the loss of this singlet was observed in the 1H-NMR spectrum of 2a (Figure S3).
3. Materials and Methods
3.1. General
The major chemicals, such as copper (I) chloride, copper (I) iodide, copper (I) bromide, TMEDA (N,N,N′N′-tetramethylethylenediamine), tetrachloromethane, tetrahydrofuran, acetonitrile, ethylene glycol, ethanol and methanol, were purchased from Qingdao Sigma Chemical Co., Ltd. (Qingdao, China). First, 1a [19] was synthesized according to the literature procedures and its structure was proved by the 1H and 13C-NMR spectra. All reagents were used at reagent grade or used after distillation. Additionally, solvents were dried by standard methods before use. All reactions were carried out by stirring in the magnetic stirrer under atmospheric air condition, and the reaction progress was the identity of the synthesized compounds controlled by TLC analysis, which was performed on Merck Silica gel 60 GF254 plates. Column chromatography was performed on silica gel and visualization in UV light. IR spectra of the synthesized compounds were recorded on The Thermo Scientific Nicolet iS50 FT-IR spectrometer, (Raman module, Waltham, MA, USA); 1H and 13C-NMR spectra were recorded on Bruker Avance (400.1 and 100.6 MHz, respectively) spectrometer at 20–25 °C in CDCl3, solution using the solvent line as an internal reference. Multiplicities are marked as s = singlet, d = doublet, t = triplet, q = quartet, quint = quintet, m = multiplet.
3.2. Synthesis of 2a
A one-necked 50 mL round-bottom flask (or 12 mL vial) was charged with 1a (1.0 mmol, 0.168 g), TMEDA (2 mmol, 0.255 g), CuCl (1.0 mmol) in MeOH (2 mL) in atmospheric air conditions. The reaction mixture was kept at 20–30 °C under stirring for 30 min using an ultrasonic irradiation bath. CCl4 0.2 mL was added slowly and mixed for 12 h on the magnetic stirrer. Volatiles were evaporated in vacuo, and the residue was purified by column chromatography using appropriate mixtures of hexane and CH2Cl2 (3:1 followed by 1:1 and 0:1) as eluents. Slightly brown oil, yield 0.274 g (82%). 1H-NMR (400.1 MHz, CDCl3): 2.02 (s, 2H, 2OH), 1.71–1.59 (m, 4H, 2CH2), 1.52–1.40 (m, 10H, 2CH2, 2CH3), 1.34–1.21 (m, 16H, 8CH2), 0.87 (t, 6H, 2CH3, J = 6.9 Hz); 13C-NMR (100.6 MHz, CDCl3): δ 83.2, 68.7, 67.4, 43.5, 31.8, 29.6, 29.5, 29.2, 24.6, 22.6, 14.1. IR: 3367 sm−1 (−OH), 2954–2855 sm−1 (–CH2), 2216 sm−1 and 2046 sm−1 (−1≡C−), 1458–1370 sm−1 (–CH2), 1307-1253 sm−1 (–CH2), 1220–1098 sm−1 (C−O−H), 1016–733 sm−1 (–CH2). HRMS (ESI-TOF): m/z [M − H]+ Calcd for C22H37O+: 317.2839; found: 317.2848.
4. Conclusions
For the first time, the dimerization reaction of acetylene alcohol 1a was carried out in the CuCl/TMEDA/CCl4/MeOH catalytic system. 8,13-Dimethylicosa-9,11-diene-8,13-diol was synthesized with 82% yield. Alternative conditions for the dimerization reaction were determined by studying the effects of copper (I) halides, N,N,N′N′-tetramethylethylenediamine and solvents.
Supplementary Materials
The following supporting information for the characterization of 1a and 2a can be downloaded online: Synthesis and reaction scheme of 1a and 2a (Scheme S1: Synthesis of 3-methyldec-1-yn-3-ol). Figure S1: 1H-NMR spectrum of 1a (400 MHz, CDCl3); Figure S2: 13C-NMR spectrum of 1a (101 MHz, CDCl3); Figure S3: 1H-NMR spectrum of 2a (400.1 MHz, CDCl3); Figure S4: 13C-NMR spectrum of 2a (100.6 MHz, CDCl3); Figure S5: IR spectrum of 2a (The Thermo Scientific Nicolet iS50 FT-IR spectrome-ter, Raman module, Waltham, MA, USA).
Author Contributions
Conceptualization, O.E.Z. and V.M.M.; methodology, V.M.M. and S.I.T.; validation, V.M.M., O.E.Z. and S.I.T.; formal analysis, V.M.M., O.E.Z. and S.I.T.; investigation, V.M.M.; resources, V.M.M.; data curation, S.I.T.; writing—original draft preparation, O.E.Z., S.I.T. and A.B.P.; writing—review and editing, O.E.Z., S.I.T. and A.B.P.; supervision, O.E.Z.; project administration, O.E.Z. and V.M.M.; funding acquisition, S.I.T. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the National Governmental Foundation “EL-YURT UMIDI” of Uzbekistan for their financial support.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Department of Organic Chemistry of Moscow State University in Russian Federation and Chirchik State Pedagogical University in Uzbekistan Republic for their technological support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tan, R.K.; Liu, Y.; Xie, L. Reinforcement Learning for Systems Pharmacology-Oriented and Personalized Drug Design. Expert Opin. Drug Discov. 2022, 17, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-J.; Kwon, H.-S.; Kang, M.; Leem, H.; Lee, K.-H.; Kim, D.-Y. The Antitumor Natural Compound Falcarindiol Disrupts Neural Stem Cell Homeostasis by Suppressing Notch Pathway. Int. J. Mol. Sci. 2018, 19, 3432. [Google Scholar] [CrossRef] [PubMed]
- Li, W. Isolobetyol, a New Polyacetylene Derivative from Platycodon grandiflorum Root. Nat. Prod. Res. 2022, 36, 466–469. [Google Scholar] [CrossRef]
- Chung, C.-Y.; Yang, W.-C.; Liang, C.-L.; Liu, H.-Y.; Lai, S.-K.; Chang, C.L.-T. Cytopiloyne, a Polyacetylenic Glucoside from Bidens Pilosa, Acts as a Novel Anticandidal Agent via Regulation of Macrophages. J. Ethnopharmacol. 2016, 184, 72–80. [Google Scholar] [CrossRef]
- Geng, C.-A.; Huang, X.-Y.; Chen, X.-L.; Ma, Y.-B.; Rong, G.-Q.; Zhao, Y.; Zhang, X.-M.; Chen, J.-J. Three New Anti-HBV Active Constituents from the Traditional Chinese Herb of Yin-Chen (Artemisia scoparia). J. Ethnopharmacol. 2015, 176, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Fois, B.; Bianco, G.; Sonar, V.P.; Distinto, S.; Maccioni, E.; Meleddu, R.; Melis, C.; Marras, L.; Pompei, R.; Floris, C.; et al. Phenylpropenoids from Bupleurum fruticosum as Anti-Human Rhinovirus Species A Selective Capsid Binders. J. Nat. Prod. 2017, 80, 2799–2806. [Google Scholar] [CrossRef]
- Liu, X.; Latkolik, S.; Atanasov, A.; Kunert, O.; Pferschy-Wenzig, E.-M.; Heiss, E.; Malainer, C.; Schinkovitz, A.; Kollroser, M.; Dirsch, V.; et al. Bupleurum Chinense Roots: A Bioactivity-Guided Approach toward Saponin-Type NF-ΚB Inhibitors. Planta Med. 2017, 83, 1242–1250. [Google Scholar] [CrossRef]
- Chan, G.G.; Koch, C.M.; Connors, L.H. Blood Proteomic Profiling in Inherited (ATTRm) and Acquired (ATTRwt) Forms of Transthyretin-Associated Cardiac Amyloidosis. J. Proteome Res. 2017, 16, 1659–1668. [Google Scholar] [CrossRef]
- Xu, W.-J.; Li, J.-H.; Zhou, M.-M.; Luo, J.; Jian, K.-L.; Tian, X.-M.; Xia, Y.-Z.; Yang, L.; Luo, J.; Kong, L.-Y. Toonasindiynes A-F, New Polyacetylenes from Toona Sinensis with Cytotoxic and Anti-Inflammatory Activities. Fitoterapia 2020, 146, 104667. [Google Scholar] [CrossRef]
- Christensen, L.P. Bioactive C17 and C18 Acetylenic Oxylipins from Terrestrial Plants as Potential Lead Compounds for Anticancer Drug Development. Molecules 2020, 25, 2568. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Matsuura, D.; Kanatani, H.; Yano, S.; Tsunakawa, M.; Matsuyama, S.; Shigemori, H. Inhibitory Effects of Polyacetylene Compounds from Panax ginseng on Neurotrophin Receptor-Mediated Hair Growth. Biol. Pharm. Bull. 2017, 40, 1784–1788. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.-Y.; Yang, C.-T.; Pu, X.-Y.; Fu, G.; Wang, W.; Li, Y.-X.; Feng, L.; Niu, H.-R.; Tan, J.-L.; Huang, X.-Z. Polyacetylenes from the Roots of Aralia Dumetorum. Rec. Nat. Prod. 2019, 13, 424–428. [Google Scholar] [CrossRef]
- Siemsen, P.; Livingston, R.C.; Diederich, F. Acetylenic Coupling: A Powerful Tool in Molecular Construction. Angew. Chem. Int. Ed. 2000, 39, 2632–2657. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Reddy, K.B.; Gayathri, K.U.; Prasad, A.R. Glaser Oxidative Coupling in Ionic Liquids: An Improved Synthesis of Conjugated 1,3-Diynes. Tetrahedron Lett. 2003, 44, 6493–6496. [Google Scholar] [CrossRef]
- Liao, Y.; Fathi, R.; Yang, Z. Aliphatic Acetylenic Homocoupling Catalyzed by a Novel Combination of AgOTs−CuCl 2 −TMEDA and Its Application for the Solid-Phase Synthesis of Bis-Benzo[b]Furan-Linked 1,3-Diynes. Org. Lett. 2003, 5, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Atobe, S.; Sonoda, M.; Suzuki, Y.; Yamamoto, T.; Masuno, H.; Shinohara, H.; Ogawa, A. Palladium-Catalyzed Oxidative Homocoupling Reaction of Terminal Acetylenes Using Trans-BidentaTable 1-(2-Pyridylethynyl)-2-(2-Thienylethynyl)Benzene. Res. Chem. Intermed. 2013, 39, 359–370. [Google Scholar] [CrossRef]
- Zhu, B.C.; Jiang, X.Z. A New CuAl–Hydrotalcite Catalyzed Homocoupling Reaction of Terminal Alkynes at Room Temperature. Appl. Organomet. Chem. 2007, 21, 345–349. [Google Scholar] [CrossRef]
- Zheng, Q.; Hua, R.; Wan, Y. An Alternative CuCl–Piperidine-Catalyzed Oxidative Homocoupling of Terminal Alkynes Affording 1,3-Diynes in Air. Appl. Organomet. Chem. 2009, 24, 314–316. [Google Scholar] [CrossRef]
- Hosseini, A.; Seidel, D.; Miska, A.; Schreiner, P.R. Fluoride-Assisted Activation of Calcium Carbide: A Simple Method for the Ethynylation of Aldehydes and Ketones. Org. Lett. 2015, 17, 2808–2811. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).