1. Introduction
Selenium cyanides are a class of inorganic compounds restricted to Se(CN)
2, Se
2(CN)
2 and Se
3(CN)
2 [
1,
2,
3,
4,
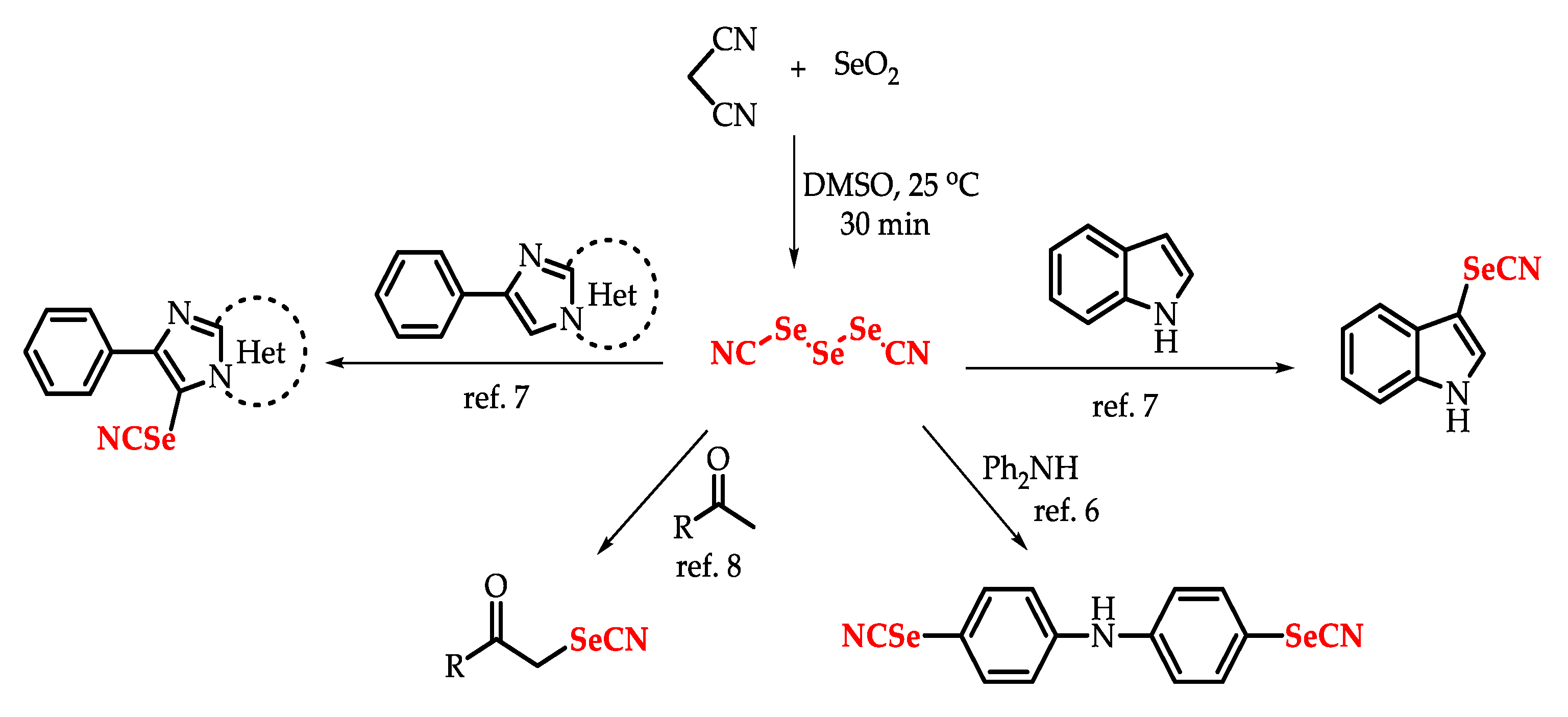
5]. Among them is triselenium dicyanide (TSD), which demonstrates a synthetic utility in organic synthesis by allowing the electrophilic SeCN moiety to be inserted into more complex structures (
Scheme 1) [
6,
7,
8], and can be used for medicinal chemistry (
Scheme 1).
For example, Kachanov and co-workers developed in 2004 a simple method for the introduction of the SeCN moiety in different aromatic amines containing free
para-position indoles and some active methylene as substrates, furnishing the corresponding selenocyanates compounds in good yields [
6]. More recently, Myrboh and co-workers reported a direct and efficient one-step protocol for the selenocyanation of aryl methyl ketones/styryl methyl ketones by selenium dioxide and malononitrile to obtain the desired α-carbonyl selenocyanates, where TSD was employed as a selenocyanating agent formed in situ [
8].
Although the in situ generation of TSD represents a fast and efficient pathway by which to insert the SeCN moiety [
9], to expand its use in organic synthesis, it is necessary to access the isolated form, mainly due to the presence of unreacted SeO
2 in the reaction medium of the one-pot approach. Consequently, the thermal and moisture sensitiveness of TSD can affect the purification step and the isolated yield and prevent mass and NMR characterizations. In this sense, an X-ray diffraction study of TSD confirmed its triselenide bridge structure, in which it was observed that the CN groups are eclipsed [
10]. However, X-ray is not an experimental protocol suitable for purity assessment and/or reaction monitoring.
TSD can be easily prepared via the exothermic reaction between malononitrile and selenium dioxide using DMSO as a solvent [
6]. However, no further characterization has been obtained due its poor solubility in organic solvents, [
10] and only X-ray crystal and solid-state nuclear magnetic resonance (NMR) analyses have been described [
4,
5,
10,
11]. On the other hand, Se(CN)
2 is stable, while Se
2(CN)
2 rapidly disproportionates [
6,
11]. Additionally, there are
77Se and
13C NMR analyses of Se(CN)
2 and Se
2(CN)
2 species, but they were collected at low temperatures [
10,
12], which hamper a selenium chemical shift comparison for a mechanism evaluation and product identification.
In view of these limitations regarding the chemistry of selenium cyanides, and because most of the reactions with TSD have been carried out at room temperature [
6,
7,
8], we report here the characterization of Se(CN)
2, Se
2(CN)
2 and Se
3(CN)
2 by
77Se and
13C NMR spectroscopies in DMSO-d
6 solvent at room temperature (25 °C). In order to better understand the practical synthetic methodology used to access TSD from the reaction between malononitrile and selenium dioxide, we also evaluated the solubility and purification step by measuring the TSD melting point (MP) and collecting infrared (IR) absorption data.
3. Discussion
The use of NMR spectroscopy to follow a reaction is an effective way to provide evidence for a mechanism [
13,
14]. NMR can provide structural details about the reactions, visualizing the consumption of starting materials and/or the formation of products, along with kinetic information obtained from the easy quantitative NMR evaluation. Additionally, to obtain more information regarding the reactivity of selenium species,
77Se nuclide in NMR analysis is an important tool to for the structural elucidation of products and chemical intermediates [
14]. Based on these features, our main idea was to acquire information on the
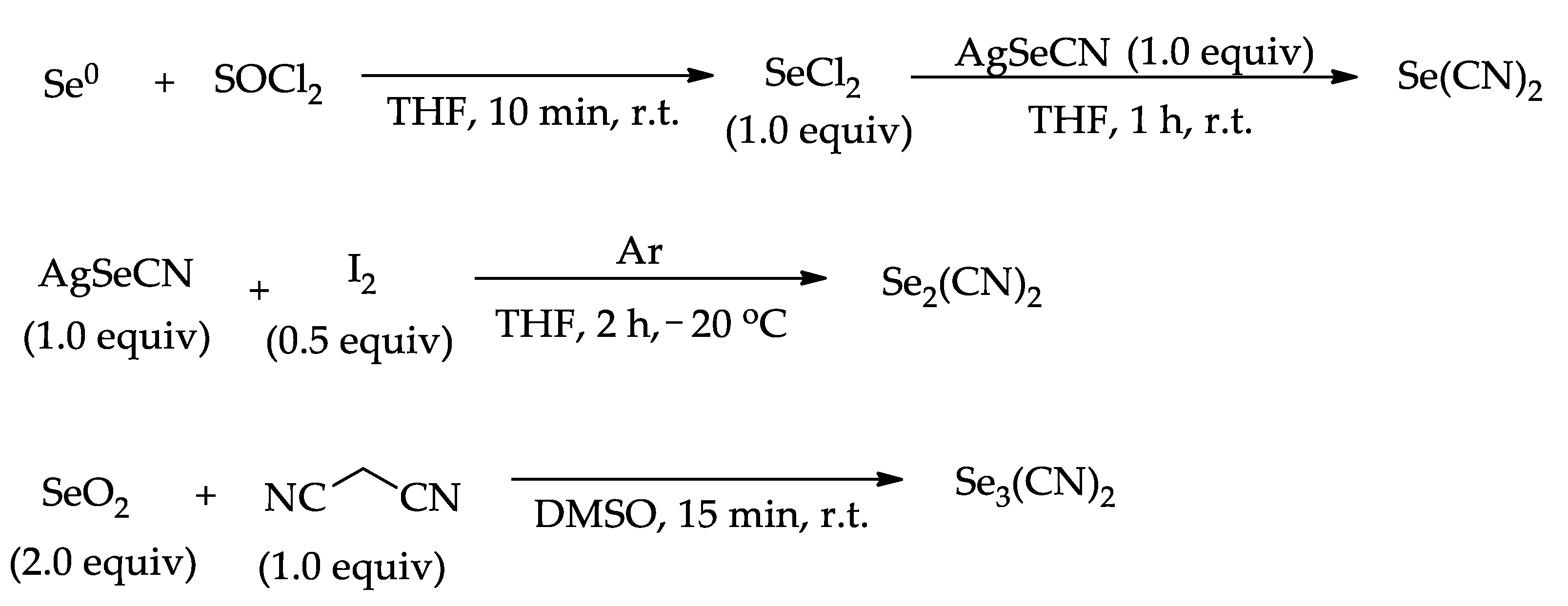
77Se NMR chemical shifts of selenium cyanides, especially TSD, once it could be used to understand the reactivity of this species in the functionalization of heterocycles (
Scheme 1) [
6,
7,
8]. For this purpose, we prepared TSD, employing SeO
2 (0.34 mmol) in the reaction with malononitrile (0.17 mmol) in 750 µL of DMSO-d
6 (
Scheme 2) [
6], and then the
77Se{
1H} NMR experiment was performed (
Figure 1). It is important to mention that the NMR sample of TSD was centrifuged to eliminate the elemental selenium residue (
Figure S1).
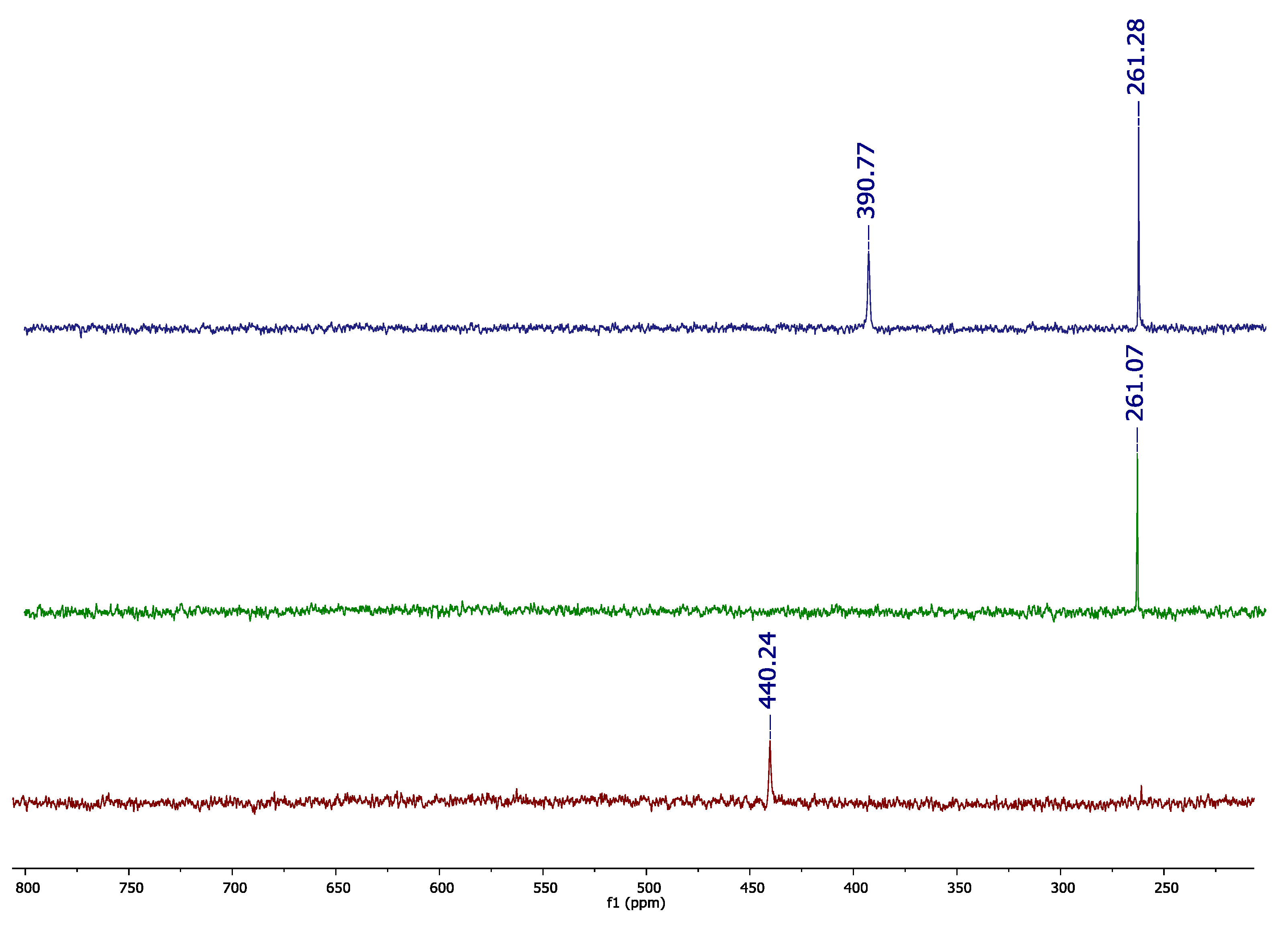
The recorded TSD
77Se{
1H} NMR spectrum presented two signals, at δ = 390.7 and 261.3 ppm, which characterize the two types of selenium-77 nuclides in Se
3(CN)
2 (
Figure 1). In the
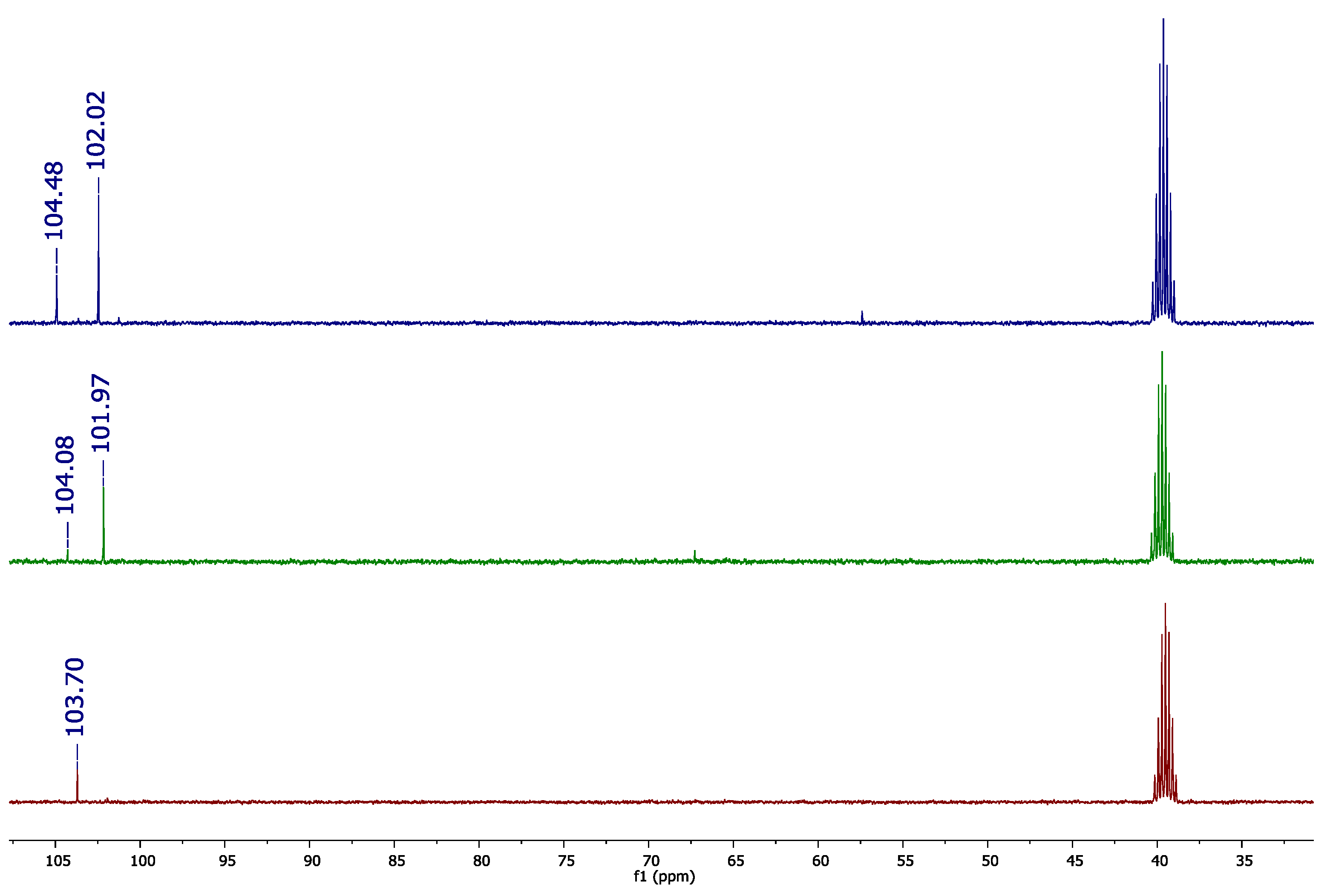
13C{
1H} NMR spectrum, we observed a signal of carbon-13 at δ = 102.0 ppm; however, another lower signal at δ = 104.0 ppm was also present (
Figure 2). To understand the
77Se and
13C NMR profiles, Se(CN)
2 and Se
2(CN)
2 were prepared and analyzed using NMR spectroscopy (
Scheme 2) [
10]. At the beginning, we performed the
77Se{
1H} NMR experiments for these selenium cyanide compounds using DMSO-d
6 as a solvent. Se(CN)
2 presented a selenium-77 signal at δ = 440.6 ppm, while Se
2(CN)
2 demonstrated a signal at δ = 261.1 ppm (
Figure 1). As can be seen in the
77Se NMR spectra, the lower signal frequency of Se
3(CN)
2 was similar to that of the Se
2(CN)
2 compound, while the higher signal frequency was not close to that observed in the selenium-77 chemical shift of the Se(CN)
2 species.
Next, the
13C{
1H} NMR spectra of Se(CN)
2 and Se
2(CN)
2 were acquired (
Figure 2). The
13C-NMR spectrum of Se(CN)
2 presented a signal at δ = 103.7 ppm, while Se
2(CN)
2 showed two signals, at δ = 101.97 and 104.08 ppm. According to the literature, Se
2(CN)
2 disproportionates to the Se(CN)
2 species, which can be visualized by the presence of the peak at δ = 104.08 ppm, due the higher stability of Se(CN)
2 [
10]. Considering that Se
2(CN)
2 has an identical selenium-77 chemical shift with Se
3(CN)
2 (~261 ppm,
Figure 1), we assume that the carbon-13 signal at δ = 104.48 ppm in the TSD spectrum was due to the presence of Se(CN)
2, once the chemical shifts were similar. Additionally, other possible structures involving isoselenocyanides can be excluded, as observed by infrared (IR) spectroscopy, in which there were no other CN bands (
Figures S10–S12) [
11]. TSD was isolated, and then the solubility was checked in CDCl
3, D
2O, Py-d
5, benzene-d
6, D
3COD and D
3CCN deuterated solvents. Although TSD was soluble only in D
3COD and D
3CCN solvents, the
77Se{
1H} NMR experiment of TSD in D
3COD did not show signals, and the solution color changed to red (
Figure S2). The
77Se{
1H} NMR spectrum of TSD in D
3CCN demonstrated a variation in the selenium-77 chemical shifts in both
77Se NMR signals for a higher frequency region than in DMSO-d
6 (
Figure S9: δ = 452.8 and 279.4 ppm).
Although it was possible to characterize all selenium cyanides, the purity of TSD should be evaluated before its proper use as a starting material in organic synthesis. An alternative protocol to check the purity of the TSD compound involves its melting point (MP), which is a practical procedure with a low cost and is properly described in the literature (133–134 °C) [
6]. In this sense, TSD was prepared according to Kaminskii and co-workers’ procedure [
6], and then different purification protocols were evaluated, as can be seen in
Table 1. According to the melting point study, the crude product had a distinct MP (
Table 1, entry 1), possibly due to the presence of Se(CN)
2 along with the Se
3(CN)
2 starting material, as visualized in the
77Se{
1H} NMR spectrum. When the yellow solid was washed with ethanol, the solid became red, exactly as observed in the solubility study, and the measured MP was 132–135 °C (
Table 1, entry 4). The solid obtained after washing with water and dichloromethane also presented distinct MPs (
Table 1; entries 2 and 5). Only after recrystallization with benzene did the obtained TSD present the correct MP (
Table 1, entry 3).
Regarding the MP measures, small variations in their values were not sufficiently precise to assess the purity of the prepared TSD. Thus,
77Se NMR analyses were performed as well to confirm the purity of the TSD after purification. As can be seen in the
Supplementary Materials (Figures S10 and S11), the
77Se NMR spectra collected after procedures of entries 2 and 3 (
Table 1) demonstrate a great amount of Se(CN)
2, while the methodology of entry 4 (
Table 1) did not show signals in the spectrum. Finally, when TSD was washed with dichloromethane, the
77Se{
1H} NMR spectrum confirmed the higher purity of this selenium cyanide species. Although the MP experiment can be used to evaluate the synthetic protocol used to obtain TSD (
Table 1),
77Se NMR spectroscopy is the best choice to confirm its purity.
Once selenium cyanides were synthesized, the NMR experiments were immediately performed due to their instability and/or disproportionation [
10,
11]. Moreover, the use of TSD in situ minimizes costs and time; nonetheless, depending on the synthetic condition, it could be necessary to isolate TSD. In this case, a careful purification should be carried out once TSD disproportionates easily at room temperature under heating. Additionally, the presence of impurities, such as SeO
2 (
Figures S10 and S11; selenium chemical shift
δ around 808 ppm), is observed in the purification protocols (
Table 1, entries 2 and 3); this accelerates the degradation process, during which the TSD solid rapidly becomes dark. Regarding the sensitivity to light, changes in the prepared TSD were not observed when it was exposed to light. The infrared (IR) analyses were also performed for all the selenium cyanide species, with the following vibrations for Se
3(CN)
2 being observed: strong nitrile stretching at 2130 cm
−1 and a weak band at 1646 cm
−1 along with a broad unassigned band at 3442 cm
−1 [
10], which could be due to water and/or impurities, as observed in the
13C{
1H} NMR spectrum (
Figure S8). Se(CN)
2 and Se
2(CN)
2 IR analyses corroborated the literature [
10], in which the main bands, such as CN at 2137 and 2139 cm
−1, and Se-CN at 668 and 671 cm
−1, respectively, could be observed with other band combinations.