Abstract
Herein, we report the synthesis and single-crystal X-ray structures of three (η6-p-cymene)Ru(II) tetrafluoroborate salts, viz., [(η6-p-cymene)(3-chloro-6-(1H-pyrazol-1-yl)pyridazine)Ru(X)]BF4, (X = Cl, Br, I), Ru1-3. They were prepared by the reactions of [(η6-p-cymene)Ru(μ-X)(X)]2, (X = Cl, Br, I) with two-mole equivalents of 3-chloro-6-(1H-pyrazol-1-yl)pyridazine, under inert conditions at ambient temperatures, and subsequently precipitated by the addition of excess BF4− ions. Orange crystalline precipitates were obtained in good yields, from which the respective single crystals for X-ray diffraction analysis were recrystallized by slow evaporation from their methanolic/diethyl ether solutions. The Ru(II) complexes were characterized by various spectroscopic techniques and chemical methods, which included FTIR, 1H/13C NMR, UV-visible absorption, mass spectrometry, and elemental analysis. The molecular structures were solved by single-crystal X-ray crystal diffraction analysis. The complexes crystallized in the monoclinic crystal system in the P21/c (Ru1-2) and P21/n (Ru3) space groups. Density Functionals Theoretical (DFT) calculations were performed in methanol to gain an understanding of the electronic and structural properties of the complexes. Trends in the data metrics were established, and selected data were compared with the diffraction data. The electrophilicity indices of Ru1-3 follow the order Ru3 > Ru2 > Ru1, and the trend is in line with their anticipated order of reactivity towards nucleophiles.
1. Introduction
Reactions of suitable precursor complexes of iridium (Ir), rhodium (Rh), ruthenium (Ru), or osmium (Os) with η6-arene ligands form chemically stable halogen-bridged intermediates, from which the monomeric half-sandwich- or piano stool-structured complexes, [(η6-arene)M (κ2-N,N′-L)X]Y (M = Ir(I), Rh(1), Ru(II), or Os(II); X = halide; Y = a counter anion, and N,N′-L = bidentate with N-donor atoms) are formed by the 1:2-mole ratio reaction between the respective intermediates with the N,N′-bidentate ligand [1]. These reactions proceed under mild conditions to form the half-sandwich complexes in good yields. The π-face coordinated η6-arene ligand brings extra chemical stability to the complexes. It π-donates its ring electrons to the metal center, forming three strong M-C bonds. It also confers necessary hydrophobicity to the coordination shell, stabilizes the oxidation state of the metal ion, as well as modulates the lability of the co-ligand. Consequently, the reactivity of the complex towards substitution of the labile co-ligand by nucleophiles also depends on the electronic properties of the coordinated η6-arene non-leaving ligand. Most of these cationic complexes have sufficient aqueous solubility despite the hydrophobicity of the η6-arene and ancillary ligand, L. These complexes, therefore, exhibit a suitable hydro-lipophilicity balance, which lends them suitable for many biomedical applications [2].
Current research efforts are aimed at developing Ru(III/II) complexes as alternatives to Pt(II) anticancer metallodrugs [3,4,5,6,7,8,9,10,11]. Ru(III) complexes such as NAMI-A, KP1019, and many other cytotoxic Ru(III) complexes [7,9,10] are thought to be anticancer prodrugs. Under the anoxic conditions of most cancer cells, they are reduced to their more reactive and active Ru(II) analogues [8]. Recently, promising cytotoxic Ru(II) complexes bearing an η6-arene, an ancillary, and a chloro (as the labile ligand) have been developed. Lead cancer cytotoxic examples in this group include RAPTA-C [12,13] and RM175 [3]. The former bears a bulk 1,3,5-triaza-7-phosphaadamantane (PTA) as the ancillary ligand, while an N,N′ bidentate (ethylenediamine) ligand features in the latter. These exemplary η6-arene Ru(II) complexes exhibit equivalent and in some cases better antitumor activities, reduced toxicity, low cross-resistance to cisplatin-resistant (CisRPt) cell lines, and better aqueous solubility. The aforementioned chemical properties place η6 arene Ru(II) complexes at the center stage for concerted research to attain more effective chemotherapeutic regimens.
The seminary work of Sadler et al. [14] on the effect of the leaving ligand on the cancer antiproliferation activity of the [(η6-arene)(azo/aminopyridine-κ2-N,N′)Ru/Os(II)X]+, (X = Cl, I) complexes was a landmark testimony to the impact of the co-ligand on cytotoxicity. The complexes were designed to have the halide (Cl−, I−) as the activation site. Their rate of substitution by bio-nucleophiles (and hence the cytotoxic activity) would be modulated by the non-leaving ligands. The iodo analogues were found to be more cytotoxic compared to their chloro counterparts. The former analogues had better cellular accumulation profiles (in membranes and associated organelles) than the latter and were less cross-resistant to a CisRPt cell line. The results demonstrated how a careful choice of the leaving group in the η6-arene Ru(II) complexes can be useful for fine-tuning the pharmacological kinetics, cellular accumulation rates, and subsequent compartmentalization. These factors were attributed cooperatively to the enhanced chemotherapeutic effect for the iodo analogues.
In this work, three η6-arene ruthenium(II) complexes of the form [(η6-p-cymene)(3-chloro-6-(1H-pyrazol-1-yl)pyridazine)Ru(X)]BF4, (X = Cl, Br, I), Ru1-3, were synthesized. Their molecular structures are shown in Figure 1. The aromaticity of the 6-(pyrazol-1-yl)pyridazine, (pzn-pdzn) is stabilized by electron-withdrawing groups such as the chloro on the 3-position of the pdzn ring in the coordinated bidentate of the synthesized complexes [15]. Unlike pyridine (pKa = 5.3), which is known to be a strong base and strong acceptor of electron density from metal-centered d-orbitals, the donor/acceptor properties of pdzn as a ligand are not well understood. Pdzn is a popular pharmacophore sub-structure of several therapeutic drugs [16]. Examples include cefozopran, cadralazine, minaprine, pipofezine, and hydralazine. All these were the pull factors in choosing pdzn as a substructure on one of the non-leaving ligands of the studied Ru(II) complexes. The substitutional lability of the complexes was varied by changing their halide co-ligand to study the correlation between the reactivity of these complexes. We report and discuss the synthesis and single crystal X-structures of these complexes. Geometry-optimized data computed at the Density Functional Theory (DFT) level were compared with the crystal structure data and employed to give insight into the electronic and structural properties of complexes.
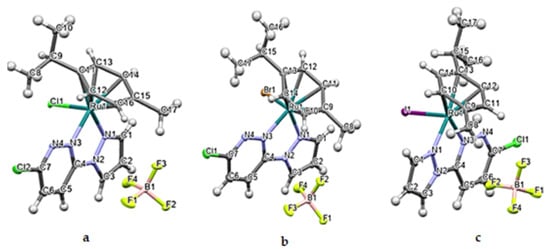
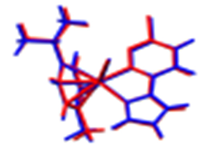
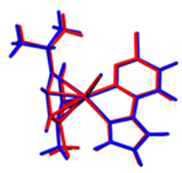
Figure 1.
The Ortep diagrams of the Ru(II) complexes, [(η6-p-cymene)(3-chloro-6-(1H-pyrazol-1-yl)pyridazine)Ru(X)]BF4, (a) Ru1 (X = Cl); (b) Ru2 (X = Br); (c) Ru3 (X = I), showing their asymmetric units. The displacements of atoms are shown at the 50% probability level.
2. Results and Discussion
2.1. Crystal Structures of Ru1-3
Suitable single crystals of Ru1-3 for X-ray diffraction measurements were afforded by slow evaporations of concentrated methanol/diethyl ether (MeOH/Et2O) solutions, and their molecular structures were solved by crystallographic analysis. The thermal ellipsoids (at 50% probability) depicting the molecular structures of the three complexes are shown in Figure 1. Data on the cell dimensions and other details on the data collection, refinement, and key structural parameters are presented in Table 1. Selected bond lengths and angles are presented in Table 2.

Table 1.
Summary of the crystal metric data for Ru1-3.

Table 2.
Selected bond length/Å and angles (°) from the crystal data for Ru1-3.
Complexes Ru1-3 were crystallized from their respective MeOH/Et2O solutions as non-solvated salts comprising the respective cation of the general formula [(η6-p-cymene)(3-chloro-6-(1H-pyrazol-1-yl)pyridazine)Ru(X)]+ (X = Cl, Br, I), and BF4− as the counter anion. The structures are as depicted in 1. All three complexes assume a pseudo-octahedral geometry, which is widely reported for η6-arene Ru(II) half-sandwich-shaped complexes [11,12,14,17]. Characteristic of these complexes is the facial π-coordination of the η6-arene, leaving three (for octahedral) and in some cases two (for trigonal-/square pyramidal) coordination vacancies on the d6 Ru(II) metal ion. The arene for these Ru(II) complexes is η6-cymene (η6-cym) and forms three nearly equivalent Ru-C π-bonds. The Ru to η6-cym centroid bond distances are 1.676 Å (Ru1-2) and 1.679 Å (Ru3), respectively, and are within the range reported for similar structured Ru(II) complexes [18]. These short centroid bond distances and the strong Ru-C π-bonds render them robust complexes with remarkable thermodynamic stability against hydrolysis or nucleophilic attack under ambient or aqueous conditions. These attributes make η6-arenes appealing ligands for stabilizing d6 organometallic complexes for various applications, including in the biomedical field [11,12,14,16,17]. The remaining coordination vacancies are occupied by the halide as the labile co-ligand, and the 3-chloro-6-(1H-pyrazol-1-yl)pyridazine bidentate via its N3pdzn, N1pzn donor atoms. The latter forms a five-membered chelate ring with the Ru(II) ion.
Coordination inorganic chemists are enchanted by the geometric distortions (conformational arrangement) of the octahedral η6-arene-bearing complexes of Ru(II) such as Ru1-3. They liken the distortion of the geometry to a ‘piano stool’. The facially coordinated η6-cym is akin to the illusory ‘seat’ of the stool. The halide (X = Cl, Br, or I for Ru1-3), the N3pdzn, N1pzn donor arms/atoms of the bidentate, are conformationally pronged onto the feet of the pseudo legs of the stool [5,6,14,17,18,19,20,21]. Despite this dramatic distortion from the perfect octahedral geometry, these η6-arene complexes have sufficient chemical and thermal stability.
The pzn and the pdzn rings of the N,N′-bidentate ligand remain coplanar upon their chelation to the Ru(II) ion. The N1-N2-C4-N3 torsion angles are near zero and equal to −0.1(9)°, −0.8(3)°, and −5.4(3)° for Ru1-3, respectively. The Ru-N bond lengths of the compounds lie between 2.0712(17) and 2.080(2) Å, which are similar to those reported for other η6-arene Ru(II) complexes with N,N′ donor ligands [5,6,14,17,18,19,20,21]. The N-Ru-N bond angles range from 76.18(9)° to 76.33(9)°, while the N-Ru-X bond angles range from 83.50(5)° to 88.44(6)°. These ranges are similar to those reported for related η6-arene Ru(II) complexes with N,N′ bidentate ligands [20]. The Ru-X bond lengths increase as expected in the order Ru-Cl (2.3920(6) Å) [14,17,18,19], Ru–Br (2.5228(2) Å [11,22], and Ru–I (2.6996(2) Å) [11,14,23] for Ru1-3 in line with the size of the labile ligand and the increase in the polarizability of the Ru-X bond. The bond lengths are comparable to those reported for related compounds: Ru–Cl [24], Ru–Br [22], and Ru–I [23]. The lengths and angles of all C—C, C—N, and B—F bonds are within the expected ranges for (N,N/O-bidentate)(η6-cym)Ru(II) complexes [3]. There are many inter/intramolecular interactions in the solid state of these Ru(II) borate salts (refer to Table S11 for a list of selected stabilizing short contacts or non-conventional hydrogen bonds). Some of these interactions occur between fluorine and hydrogen atoms of p-cym. Examples are the F…H–C short contact bonds (e.g., C17–H17···F3B, d = 2.868(4) for Ru1, while for Ru2 and Ru3, similar non-conventional hydrogen bonds occur between the leaving group (X) and the hydrogen atom of the neighboring cation in the lattice, as exemplified by the C–H17…Br1 = 3.024 Å and C16-H16…I1 = 3.67 Å. A noteworthy difference in the relative conformational geometry and orientation of the ligands around the Ru(II) metal center is that the pzn and pdzn rings of Ru3 are coordinated in contrastingly distinct orientations (when referenced to the orientations of substituents on the cymene) to that of the same rings in Ru1-2, as well as the analogue of Ru1 reported in [18]. It is unclear what drives the difference in the orientations of the two non-leaving ligands which incidentally put these complexes in two coordinational or configurational sub-domains. In Ru3, the propyl group of the p-cym is oriented closer to the larger ring of the pdzn ligand, which seems to increase electron–electron repulsion between the groups, leading to a longer Ru-CCym(centroid) distance for Ru3 relative to that of either Ru1 or Ru2. Contrastingly, the methanolic solutions afforded orange crystalline salts of Ru1-3. This is an indication that the coordinated halide ligand has little or no influence on the strength of the crystal field, and this is supported by the invariance in the magnitude of the HOMO-LUMO energy band gap (Table 3). The trend in the DFT-calculated data shows that there is no significant difference in the band gap energies of the three complexes. The commonality in the color of the salts of homologous halo-Ru(II) complexes was also reported for a series of [(η6-arene)Ru(II)]N-N′)X]+, X = Cl, Br and I complexes [11]. Only when the counter ion in the analogous salts of Ru1 was changed from BF4− to PF6− was there a distinct color change from orange to yellow [18].

Table 3.
DFT-calculated data for complexes Ru1-3.
2.2. Spectroscopic Data
The molecular structures of the N,N′-bidentate ligand and its complexes Ru1-3 in solution were probed by 1H/13C NMR spectra and 2D-NMR techniques (correlation spectroscopy (COSY) and the heteronuclear single quantum coherence (HSQC)). NMR data are useful in elucidating the molecular structure behavior of compounds in solution. The 1H/13C NMR data for the ligand and the complexes are listed in the experimental section and their respective spectra are given in Figures S2–S7 (Supplementary Materials), respectively. The 1H NMR resonances for the free ligand (Figure S1) appear upfield relative to those of Ru1-3. The 1H/13C NMR spectra of Ru1-3 show common chemical shifts in the resonances of the 1H and 13C nuclei. This is an indication that the variation in the halide co-ligand has little or no influence on the electronic environment of the spins of the nuclei of the two coordinated non-leaving ligands. The 3J-constants of non-singlet peaks/(hyperfine-split resonances) in the 1H NMR spectra of Ru1-3 (Figures S2–S4) were used to confirm correlated protons in distinctive chemical environments of the complexes. The 1H spectrum of Ru3 features four common resonance (absorption) peaks while Ru1 and Ru2 have three peaks in the range of 5.90–6.30 ppm. The chemical equivalence of four protons due to the C2-symmetry of the facially coordinated η6-cym is broken by spin coupling with the diastereotopic protons of methyl groups of the isopropyl moiety. Additionally, the pseudo-octahedral (piano stool) arrangement of four different ligands around the Ru(II) ion makes the latter a chiral center. Thus, the complexes will exhibit stereoisomerism that breaks the C2-symmetry of the cym and its spin resonance absorption [17]. The resonance peaks of the three protons of pzn and two of pdzn appear as five resonance peaks downfield (δ = 7.16–9.18 ppm), attributed to the 1H nuclei on the C=N, C-N, C=C groups and those of the η6-cym, respectively. As expected, these peaks were slightly deshielded compared to that of the free ligand (Figure S1), which ranges between 6.69 and 8.80. The aliphatic protons of the cym appear at 2.79 ppm (m, 1HCH(CH3)2), 2.12 ppm (s, 3Hmethyl), and 1.15 ppm (dd, 2 × 3Hmethyl isopropyl). The total number of carbon atoms and the range of their chemical shifts (ppm) agree with the different possible bonding environments of the C nuclei of the ligand framework for the proposed structures. Curiously, the aromatic resonances for the η6-cym protons do not feature as two doublets as in the spectrum of the respective dimer precursors.
The 1D NMR data were corroborated by those of the COSY, HSQC, and other related correlated techniques, and these data are presented only for Ru3 (refer to Figure S9 and Table S1). The absence of the 1H/13C cross peaks for C4, C7 (N,N-bidentate) and C8, C11 (cym) in the HSQC of Ru3 (see Figure S9a, and for the carbon numbering, see Figure S8) confirms the quaternary nature of these carbons. The correlation assignments of the rest of the 1H/13C chemical shifts are consistent with the proposed structure of Ru3 and its two analogues. These assignments were further corroborated by the COSY data (Figure S9b and Table S1). In the COSY map of Ru3, common 1H/1H cross-peaks are observed between the absorption resonances of protons on the C1/C2, C2/C3 (pzn), C5/C6 (pdzn), and C9/C10, C12/C13, C15/C16, C15/C17 (cym), showing that their carbons are bonded together (Figure S9a). Data of the correlated protons and their mutual coupling constant (3J) are given in Table S1.
The chemical purity of Ru1-3 was checked by elemental (CHNS) analysis while their structural elucidation in the solution was accomplished by various spectroscopic techniques. The elemental compositions of Ru1-3 were within 5% of their theoretically calculated data (see the elemental data listed in the experimental section). This confirmed the high purity of the crystalline salts of Ru1-3.
Direct electro-spraying of solutions Ru1-3 (in methanol/0.1% formic/formate buffer) yielded ion peaks at m/z values of 451 amu (100%), 497 amu (100%), and 543 amu (100%) in their low-resolution mass spectra (Figures S10–S12). The zoomed-out peaks (see insets in Figures S10–S12) exhibit isotopic distribution patterns that are comparable to the theoretical patterns for the respectively calculated formulas. The matched isotopic pattern for each serves as a structural fingerprint for confirming the identity (as observed in the nominal masses of the respective pseudo molecular ions of Ru1-3 when the isotopic distribution of the elements for each complex is considered). These observed mass spectral data (Figures S10–S12) are attributed to their respective pseudo-protonated molecular ions: [C17H19ClN4RuCl + H]+ (for Ru1), [C17H19ClN4RuBr + H]+ (for Ru2), and [C17H19ClN4RuI + H]+ (for Ru3).
UV–visible electronic absorption spectra of the complexes Ru1-3 in solution are depicted in Figure S13. All the electronic spectra of the complexes show similar spectral bands to those reported for other pseudo-octahedral complexes [25]. Characteristic absorption peaks include the medium-intensity bands in the region 250–260 nm, which are assigned to π–π* electronic transitions. An intense band also features in the 280–320 nm region and it is assigned to the inter-/intra-ligand π–π*/n–π* transition within the pyrazolyl-pyridazine bidentate ligand [26]. The absorption band (low intensity) in the region 450–470 nm is assigned to the Ru(II)-to-ligand charge transfer (MLCT). However, the intensities of the absorption bands for Ru3 are weaker compared to those of Ru1 or Ru2. In general, these observed spectral data are comparable to those of other [(η6-arenes Ru(II) complexes bearing an N,N′ donor chelate [25]. As already stated, solutions of Ru1-3 precipitate as orange crystalline solids. This shows that the halide ligand has little influence on the crystal field strength of the complexes. The presence of the 3-chloro-6-(1H-pyrazol-1-yl)pyridazine as a common spectator ligand and the LUMO fragment ensures an overriding metal-to-ligand charge transfer (MLCT) limited to a narrow range band gap (3.3–3.1 eV, see data in Table 3) between the three complexes. This leads to homologous halo-Ru(II) complexes with the same color.
Some of the vibrational stretching bands of Ru1-3 (Figures S15–S17) shift to longer wavelengths compared to that of the free ligand, 3-chloro-6-(1H-pyrazol-1-yl)pyridazine (refer to Figure S14). This confirms the coordination of the bidentate ligand on the metal centers. As shown in Figures S15–S17, the FTIR spectra for Ru1-3 feature common vibrational bands due to in-plane stretches of the C-C/N bonds of the p-cym and the pzn-pdzn bidentate ligands. This is an indication that the leaving halide group has minimum influence on the vibrational frequencies of the functional groups on the non-leaving ligands. The observed absorption bands are mainly due to the functional groups of the coordinated pzn-pdzn-N,N′ bidentate ligand, the p-cym ligands, and the borate counter ion. As an example, in Figure S15 (the spectrum of the Ru1), two absorption bands are observed at 3085 and 2873 cm−1; these low-intensity bands are for the νC−H stretching frequencies for the aromatic and aliphatic stretches, respectively. Another common featuring band is the 1582 cm−1 (medium, νC=N (pzn/pdzn) and νN=N (pdzn) stretch). The absorption bands of the cym occur as an intense duet at 1479 and 1404 cm−1 (intense, νC=C(cym) stretching). The bands that appear within the range 1200 and 1400 cm−1 (sharp, medium) are due to the νC=C vibration asymmetric stretches of the pzn/pdzn ring carbons. An intense absorption band is observed in the 1020–1051 cm−1 range due to the asymmetric stretching of the νB-F bond of the BF4− counter ion of Ru1-3. The sharp bands below 800 cm−1 (medium intensities) are due to the vibrational stretches of the Ru-C, Ru-Npzn/pdzn, and Ru-Cl bonds. These frequency bands occur in the range reported in the literature [5,10,14,17,19].
2.3. DFT-Calculated Optimized Structures
Geometry-optimized structures for Ru1-3 were computed in methanol as a solvent. The structures (see Table 4, first column) and the energies of the frontier molecular orbitals are presented in Table 4. Some of these DFT-calculated data were compared with the crystal structure (solid-state) and are presented in Table 3 and Table 5.

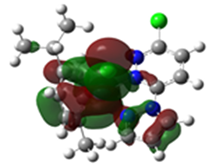
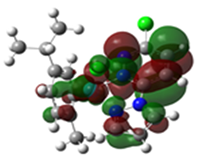
Table 4.
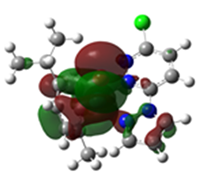
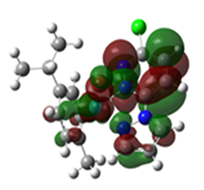
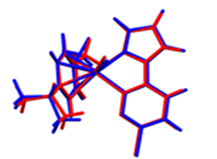
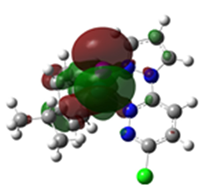
Overlays of the geometry-optimized structures on their crystal structures, and HOMO and LUMO electron density mappings for Ru1-3.

Table 5.
Comparison of selected DFT data metrics with that from the crystal structure for complexes Ru1-3.
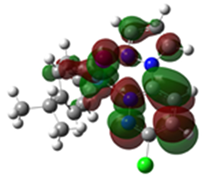
The HOMO mappings of Ru1-3 (Table 4) have a common distribution. They show significant electron density which is delocalized over the η6-cym ring, the Ru(II)-X (X = Cl, Br, I) bond, and the pzn donor arm. However, for Ru3, the density over the pzn is restricted only to the N donor atoms and is much smaller than that on the iodo ligand. For all, there is hardly any contribution to the HOMO by the pdzn ring for Ru1-2. This is as expected, since there is π- and σ-donation of electron density from their η6-cym and pzn rings, respectively. These donations reduce the effective positive charge on the Ru(II) ion, making it less electrophilic towards substituting nucleophiles. The LUMOs of Ru1-3 are more localized on the pdzn rings. Conversely, there is no significant contribution to the LUMOs from the pzn rings. Thus, the electron density on the metal can easily be back-donated into π* orbitals of the pdzn ring than into the pzn donor arm. This means that the pdzn arm of the N,N′-bidentate is a π-acceptor comparable to pyridine or pyrazine. Overall, the N,N′ bidentate of these complexes can be an effective electron density modulator through a pull-and-push mechanism towards the Ru(II) metal center since pdzn is a better π-acceptor than pzn. This is important in producing complexes that have moderate reactivity and are therefore less prone to deactivation on transit to the cellular targets. Furthermore, the energy for Ru3′s LUMO is the highest, and hence this complex is the poorest π-acceptor, and least electrophilic. However, it is likely to be the most reactive towards incoming nucleophiles because its iodo ligand is the best leaving group due to the high polarizability of its metal bond.
To probe and predict the substitutional reactivity of Ru1-3, their HOMO-LUMO band gaps and global electrochemical descriptors (chemical hardness (η) [27], chemical potential (µ), electrophilicity (ω) [28], and charge density) were compared. As the halide, X is changed from Cl (Ru1) to I (Ru3), and the band gap, electronegativity, and η decrease; thus, Ru1 is predicted to be the most stable and least reactive. The chemical potential, softness, and electrophilicity also increase from Ru1 to Ru3, which is a clear indication of the greater ability of Ru3 to accept electron density from the incoming nucleophile, thus enhancing the π-back bonding of electrons from the Ru into the π* orbital and stabilizing the transition state.
Looking at the calculated NBO charges in Table 4, the effective charge on the Ru(II) center decreases in the order Ru1 > Ru2 > Ru3. Thus, the charges on the complexes are affected by the nature of the leaving group. The Ru(II) ion in Ru1 has the highest positive charge, indicating that it is the most electrophilic. Ru3 is soft and has a high ability for accepting electrons from the soft Ru(II) ion, and hence is expected to be slightly more reactive than Ru1 or Ru2. The large size of the atomic orbitals of the iodo ligand and hence its ease of polarizability results in an instantaneously large dipole that decreases the positive charge on the Ru(II). On one hand, the chloro ligand withdraws electron density from the metal center by resonance, which increases the positive charge on Ru(II). As a result, it is not the charge on the metal that determines reactivity, but rather the general increase in the negative charge on the ligand moiety, as indicated by electrophilicity indices [28].
Overlays of the DFT optimized structures (in methanol) of Ru1-3 and their respective X-ray crystal structures show a good fit (root mean square deviation (rmsd) of < 2%) of the DFT-optimized structures. The superimposed structures are shown in the first column of Table 4. The computed length, distances, and angles are all within a 10% difference from the solid-state data (Tables S5–S10) from X-ray crystallographic analysis, thereby validating the accuracy of the theoretically calculated metric data of the complexes.
3. Materials and Methods
3.1. Reagents
The reagents and solvents used for the synthesis of the η6-p-cymene Ru(II) complexes were procured from commercial suppliers (Sigma Aldrich and Merck, Johannesburg, South Africa) and used without any further purification. The ligand 3-chloro-6-(1H-pyrazol-1-yl)pyridazine (pdzn-pzn) was prepared by following a literature procedure [29].
3.2. Synthesis of [(η6-p-cymene)(3-chloro-6-(1H-pyrazol-1-yl)pyridazine)Ru(X)]BF4, Ru1 (X = Cl); Ru2 (X = Br); Ru3 (X = I)
The complexes (Ru1-3) were all synthesized by the method of [20] with slight modifications in the purification stages. An acetonitrile solution (10 mL) of each of the precursor dimer complexes, [(η6-p-cymene)Ru(μ-X)(X)]2, (X = Cl, Br, I), was prepared by dissolving 674, 869, and 1076 mg (1.1 mmol), respectively. The solution of each was separately added to 2.2 mmol (in acetonitrile) of 3-chloro-6-(1H-pyrazol-1-yl)pyridazine [29]. The respective mixture was stirred at room temperature for 4 h under an inert atmosphere of N2 provided by the Schlenk line and accessories. This prevented the reactants from reacting with air. Thereafter, the mixture was concentrated under vacuo. About 5 mL of an ethanolic solution of NH4BF4 (2.5 mmol) was slowly added to precipitate the complexes. The mixtures were left under an ice bath for one hour, leading to the formation of orange crystalline precipitates. These were collected by filtration and washed with cold diethyl ether and dried under vacuo. The formation of the tetrafluoroborate salts is depicted in synthetic Scheme 1. Single crystals for X-ray diffraction analysis were grown by slow (1–2 weeks) diffusion of their vapors out of the methanol/diethyl ether solutions of Ru1-Ru3.
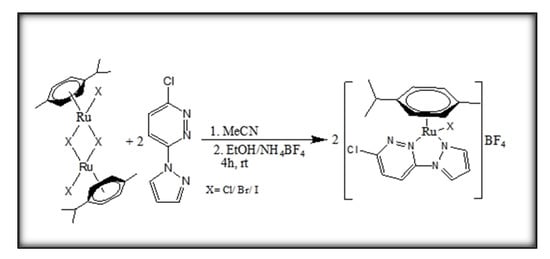
Scheme 1.
Synthetic pathways for [(η6-p-cymene)(3-chloro-6-(1H-pyrazol-1-yl)pyridazine)Ru(X)]BF4, Ru1 (X = Cl); Ru2 (X = Br); Ru3 (X = I) complexes.
3.3. Characterization of Complexes
1H and 13C NMR spectra were recorded on a Bruker Avance DPX 500 MHZ spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany) fitted with a 5 mm sample probe at 30 °C to confirm the purity and identity of the compounds. All chemical shifts of protons or carbons of 3-chloro-6-(1H-pyrazol-1-yl)pyridazine are reported relative to those of tetramethyl silane (SiMe4). The mass spectral data of the ligands and complexes were acquired on an LC Premier micro-mass spectrometer. FTIR spectra of complexes were recorded (in KBr) on the Agilent Technologies Cary 630 spectrometer (Vienna, Austria) in the 3800–600 cm−1 range. The UV–visible absorption spectra were acquired on a carry 100 Bio UV-visible spectrophotometer with a temperature controller of ± 0.05 °C. Elemental compositions (CHN) of Ru1-3 were determined on a Thermo Scientific Flash 2000 Spectrometer. The characterization data are given in the Supporting Information (SI). Single-crystal X-ray crystallographic data of Ru1-3 were collected on a Bruker APEX Duo CCD area detector diffractometer [30] with an Incoatec microsource operating at 30 W of power. The crystal was kept at a constant temperature within the range of 99.9–104 K during data collection using an Oxford Instruments Cryojet accessory (Oxford, UK). Diffraction was by graphite-monochromated Cu Kα radiation (λ = 1.54178 Å) at a crystal-to-detector distance of 50 mm. Data collection was conducted at the following set conditions: ω-/φ-scans with exposures, taken at 30 W X-ray power and 0.50 frame widths, 2θ range in the range 6.98–142.948° using SAINTS′ APEX2 [31]. The crystal structures were solved by the OLEX2 program [32], using the SHELXT [33] structure solution programs and Intrinsic Phasing and refined by SHELXL [31] refinement programs. The non-hydrogen atoms were refined anisotropically by full-matrix least-squares minimization/refinement of F2. Hydrogen atoms were included but not refined. Visualization of the crystallographic data was performed in Mercury v.4.3 (Cambridge Crystallographic Data Centre, Cambridge, UK), and the crystal data are given in Tables S2–S10.
3.4. DFT-Optimized Structures of Ru1-3
The Density Functional Theory (DFT) ground state-optimized structures of Ru1-3 were calculated using the Gaussian 09 program suite [34]. The B3LYP method [35,36], utilizing the LANL2DZ basis sets, was used to optimize the geometry of the complexes. Calculations were simulated in methanol using the conductor-like polarizable continuum model (C-PCM) [37,38].
3.5. Listed Spectral Data of Ru1-3
- 3-chloro-6-(1H-pyrazol-1-yl)pyridazine (N,N′-L): White powder, 1H NMR (500 MHz, d6-DMSO) δ (ppm), 8.80 (d, J = 2.5 Hz, 1Hpzn), 8.27 (d, J = 9.3 Hz, 1Hpzn), 8.08 (d, J = 9.1 Hz, 1Hpdzn), 7.98 (d, J = 1.4 Hz, 1Hpdzn), 6.70 (dd, J = 4.3 Hz, 1Hpzn). 13C (100 MHz, d6-DMSO): δ (ppm) 154.1 (N-C-Npdzn), 153.7 (N=C-Cl, pdzn), 143.7 (C=N, pzn), 131.8 (C=Cpdzn), 127.8, (C=Npzn), 120.9 (C=Cpdzn), 109.7 (C=Cpzn) (see also Figure S14).
- [(η6-p-cymene)(3-chloro-6-(1H-pyrazol-1-yl)pyridazine)Ru(Cl)]BF4 (Ru1): Yield 74.2%, orange crystals, MS (ESI)+ m/z (% Ipic) = 451 (100), [C17H19ClN4RuCl + H]+; 1H NMR (500 MHz, d6-DMSO) δ (ppm), 9.18 (d, J = 3.2 Hz, 1Hpzn), 9.02 (d, J = 2.2 Hz, 1Hpzn), 8.80 (d, J = 9.3 Hz, 1Hpdzn), 8.58 (d, J = 9.3 Hz, 1Hpdzn) 7.17 (dd, J = 3.2, 2.2 Hz, 1Hpzn), 6.17 (ddd, J = 12.1, 6.2, 1.1 Hz, 2HAr p-Cym), 5.99 (dd, J = 6.1, 1.1 Hz, 1H,Ar p-Cym), 5.87 (dd, J = 6.2, 1.1 Hz, 1Hp-Cym), 2.79 (p, J = 13.8, 6.9 Hz, 1Hp-Cym, CH(isopropyl), 2.12 (s, 3Hp-Cym, CH3), 1.15 (t, J = 6.9 Hz 6Hp-Cym, CH3(isopropyl). 13C (100 MHz, d6-DMSO): δ (ppm) 153.7 (N-C-Npdzn), 150.9 (C=Npzn), 148.8 (N=C-Clpdzn), 134 (C=Cpdzn), 132.9 (C=Npzn), 121.9 (C=Cpdzn), 112.8 (C=Cpzn), 105, 101.9, 86.7, 86.6, 84.2, 84.1 (CAr p-Cym), 30.3 Cp-Cym, CHisopropyl, 21.9 (-CH3p-Cym), 21.3, 17.8 (-CH3 isopropyl p-Cym). FTIR (KBr, cm−1 [(weak/medium/strong/sharp/broad = w/m/s/(shrp/br))]: 3085w(ν (Carom-H); 2959w, ν (C-Haliphatic), w(br); 1582, w(shrp), ν(N=Cpdzn/pzn); 1480, 1404, sshrp, ν(C=Ccym/pzn/,pdzn); 1284, m(shrp), ν(Ar βCHcym); 1050.37, vs(shrp), ν(BF4−); 820–850, 780, 650, mshrp ν[(Ru-Npdzn/pzn, Ru-Ccentroid cym, Ru-Cl], respectively. Elemental analysis: Calculated for [C17H19ClN4RuCl]BF4, %: C 37.94; H, 3.56; N, 10.41; found, % C, 37.82; H, 3.56; N, 10.50.
- [(η6-p-cymene)(3-chloro-6-(1H-pyrazol-1-yl)pyridazine)Ru(Br)]BF4 (Ru2): Yield 75.5%, orange crystals, MS (ESI)+ m/z (% Ipic) = 497 (100), [C17H19ClN4RuBr + H]+; 1H NMR (500 MHz, d6-DMSO) δ (ppm), 9.18 (d, J = 3.2 Hz, 1Hpzn), 8.99 (d, J = 2.1 Hz, 1Hpzn), 8.78 (d, J = 9.3 Hz, 1Hpdzn, 8.57 (d, J = 9.3 Hz, 1Hpdzn), 7.17 (dd, J = 3.2, 2.1 Hz, 1Hpzn), 6.16 (m, 2HAr-p-Cym), 5.99 (m, 1Hp-Cym), 5.88 (dd, J = 6.3, 1.2 Hz, 1Hp-Cym), 2.86 (h, J = 6.9 Hz, 1H CHisopropyl p-Cym, 2.17 (s, 3H, p-Cym, CH3), 1.17 (dd, J = 8.6, 6.9 Hz, 6H, -CH3isopropyl p-Cym). 13C (100 MHz, d6-DMSO): δ (ppm) 153.6 (N-C-Npdzn), 150.8 (C=N, pzn), 149.1 (N=C-Cl, pdzn), 133.9 (C=Cpdzn), 132.9, (C=Npzn), 121.8 (C=Cpdzn), 112.9 (C=Cpzn), 105.4, 101.8, 86.6, 84.6, 84.5Ar, p-cym, 30.3 CHisopropyl), 21.9 (-CH3p-Cym), 21.3, 17.8, (-CH3isopropyl p-Cym). FTIR (KBr, cm−1, w/m/sshrp/br (= weak/medium/strongsharp/broad): 3083, w, ν(Carom-H); 2875-3, w, ν(C-Haliphatic), w(br); 1582, w(shrp), ν(N=Cpdzn/pzn); 1480, 1403, sshrp, ν(C=Ccym/pzn/,pdzn); 1168, m(shrp), ν(Ar βCHcym); 1021, vs(shrp), ν(BF4); 820–50, 780, 650, mshrp ν[(Ru-Npdzn/pzn, Ru-Ccentroid cym, Ru-Cl], respectively. Elemental analysis: Calculated for [C17H19ClN4RuBr]BF4, %: C 35.05; %H, 3.29; N, 9.62; found, % C, 35.72; H, 3.08; N, 9.48.
- [(η6-p-cymene)(3-chloro-6-(1H-pyrazol-1-yl)pyridazine)Ru(I)]BF4 (Ru3): Yield 40%, orange crystals. MS (ESI)+ m/z (% Ipic) = 543 (100), [C17H19ClN4RuI + H]+; 1H NMR (500 MHz, d6-DMSO) δ (ppm), 9.19 (d, J = 3.2 Hz, 1Hpzn), 8.94 (d, J = 2.2 Hz, 1Hpzn), 8.78 (d, J = 9.3 Hz, 1Hpdzn, 8.55 (d, J = 9.3 Hz, 1Hpdzn), 7.17 (dd, J = 3.2, 2.2 Hz, 1Hpzn), 6.21 (dd, 1H, J = 6.4, 1.3 Hz), 6.14 (dd, J = 6.2, 1.2 Hz, 1Hp-Cym, 5.99 (dd, J = 6.4, 1.3 Hz, 1HAr-p-Cym), 5.94 (dd, J = 6.2, 1.2 Hz, 1Hp-Cym, 2.97 (h, J = 6.9 Hz, 1H, p-Cym, CHisopropyl, 2.21 (s, 3H, CH3 p-Cym), 1.19 (d, J = 11.0, 6.9 Hz, 6H, CH3isopropyl, p-Cym). 13C (100 MHz, d6-DMSO): δ (ppm) 153.5 (N-C-Npdzn), 150.3 (C=Npzn, 149.8 (N=C-Clpdzn), 133.7 (C=Cpdzn), 133 (C=Npzn), 121.5 (C=Cpdzn), 113.5 (C=Cpzn), 106.0, 102.2, 86.8, 86.5, 85.5, 84.9 (Arp-Cym), 31.1 (CH,isopropyl p-Cym, 22.8 (-CH3p-Cym, 19.1, 21.9, -CH3isopropyl p-Cym). FTIR (KBr, cm−1, w/m/sshrp/br (= weak/medium/strongsharp/broad), ν(funct. gr)): 3107, w, ν(Carom-H); 2976, w, ν(C-Haliphatic), w(br); 1581, w(shrp), ν(N=Cpdzn/pzn); 1433, sshrp, ν(C=Ccym/pzn/,pdzn); 1284, m(shrp), ν(Ar βCHcym); 1030, vs(shrp), ν(BF4); 820–50, 780, 650, mshrp ν[(Ru-Npdzn/pzn, Ru-Ccentroid. cym, Ru-Cl], respectively]. Elemental analysis: Calculated for [C17H19ClN4RuI]BF4, %: C 32.43; %H, 3.04; N, 8.90; found, % C, 32.92; H, 3.32; N, 9.07. 4.
4. Conclusions
Three borate salts of half-sandwich Ru(II) complexes, [(η6-p-cymene)(3-chloro-6-(1H-pyrazol-1-yl)pyridazine)Ru(X)]BF4 (X = Cl (Ru1), Br (Ru2), or I (Ru3)), were synthesized from the reactions of their respective dimer precursors, [(η6-p-cymene)Ru(μ-X)(X)]2 (X = Cl, Br, I), with two-mole equivalents of 3-chloro-6-(1H-pyrazol-1-yl)pyridazine under inert conditions and subsequent precipitation by the addition of excess BF4− ions. Orange crystalline precipitates were obtained in good yields, from which respective single crystals for X-ray diffraction analysis were crystallized by slow evaporation from their methanolic/diethyl ether solutions. Complete characterization of all complexes was achieved using 1H and 13C NMR, FTIR and UV-visible absorption spectroscopies, elemental analysis, and single-crystal X-ray crystallography. The η6-cym group is facially coordinated to the Ru(II) ion and occupies the apex position of the pseudo-octahedral geometry. The pyrazole and pyridazine donor arms of the ancillary N,N′-bidentate (3-chloro-6-(1H-pyrazol-1-yl)pyridazine) and a halide occupy the other sites of the piano stool geometry. DFT computed data show that the nature of the leaving group affects the effective charge electrophilicity, and hence the reactivity of the complex. Ru3 has the greatest tendency to accept the electron density of incoming nucleophiles during a ligand substitution process.
Supplementary Materials
You can access the following information online. Spectral characterization data: Figures S1–S17, including the COSY and HSQC 2D-NMR spectral data. Tables of data: Table S1, 2D-NMR data; Tables S2–S4, the atomic coordinates for Ru1-3, and Tables S5–S10, comparable isotropic displacement parameters (2 × 103), bond lengths (Å), and angles [°] for Ru1-3; Table S11, the short-contacts/non-conventional hydrogen contacts.
Author Contributions
A.K.K.: Investigation, conceptualization, synthesis, drafting; P.O.: conceptualization, partial resourcing supervision; J.G.: partial resourcing on synthesis, reviewing; R.O.O.: crystallization, spectral characterization and reviewing, L.A.H.: DFT computations, X-ray diffraction data collection, visualization, handling, and analysis; A.M.: DFT and X-ray diffraction data analyses and partial resourcing on the characterization of compounds, and drafting and reviewing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The supplementary crystallographic data for Ru1-3 (ref code: cu_am_ro_am_3a-c_0m; CDCC numbers: 2194343, 2194345, and 2194346, respectively) can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 3 August 2022), or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, U.K.; fax: (+44)1223-336-033; or via email: deposit@ccdc.cam.ac.uk.
Acknowledgments
We acknowledge the Department of Chemistry at Egerton University in Kenya, where the conception and synthesis of the complexes were carried out. This work’s partial financing is credited to the Research Office of the University of KwaZulu-Natal (UKZN), RSA. The DFT, crystallization, and single-crystal X-ray diffraction analysis were carried out at the UKZN’s School of Chemistry and Physics, PMB Campus. We thank C.J.Van Rensburg for assistance in mass spectroscopic analysis and C.Grimmer for NMR analysis.
Conflicts of Interest
No potential conflict of interest or competing interest are foreseen.
References
- Gichumbi, J.M.; Friedrich, H.B. Half-sandwich complexes of platinum group metals (Ir, Rh, Ru and Os) and some recent biological and catalytic applications. J. Organomet. Chem. 2018, 866, 123–143. [Google Scholar] [CrossRef]
- Canivet, J.; Karmazin-Brelot, L.; Süss-Fink, G. Cationic arene ruthenium complexes containing chelating 1, 10-phenanthroline ligands. J. Organomet. Chem. 2005, 690, 3202–3211. [Google Scholar] [CrossRef]
- Mendoza-Ferri, M.G.; Hartinger, C.G.; Eichinger, R.E.; Stolyarova, N.; Severin, K.; Jakupec, M.A.; Nazarov, A.A.; Keppler, B.K. Influence of the spacer length on the in vitro anticancer activity of dinuclear Ruthenium—Arene compounds. Organometallics 2008, 27, 2405–2407. [Google Scholar] [CrossRef]
- Mendoza-Ferri, M.G.; Hartinger, C.G.; Nazarov, A.A.; Eichinger, R.E.; Jakupec, M.A.; Severin, K.; Keppler, B.K. Influence of the arene ligand, the number and type of metal centers, and the leaving group on the in vitro antitumor activity of polynuclear organometallic compounds. Organometallics 2009, 28, 6260–6265. [Google Scholar] [CrossRef]
- Matveevskaya, V.V.; Pavlov, D.I.; Samsonenko, D.G.; Ermakova, E.A.; Klyushova, L.S.; Baykov, S.V.; Boyarskiy, V.P.; Potapov, A.S. Synthesis and structural characterization of half-sandwich arene–ruthenium(II) Complexes with bis(imidazol-1-yl)methane, Imidazole and Benzimidazole. Inorganics 2021, 9, 34. [Google Scholar] [CrossRef]
- Gichumbi, J.M.; Friedrich, H.B.; Omondi, B.; Lazarus, G.G.; Singh, M.; Chenia, H.Y. Synthesis, characterization, anticancer and antimicrobial study of arene ruthenium(II) complexes with 1,2,4-triazole ligands containing an α-diimine moiety. Z. Naturforsch. B 2018, 73, 167–178. [Google Scholar] [CrossRef]
- Rademaker-Lakhai, J.M.; Van Den Bongard, D.; Pluim, D.; Beijnen, J.H.; Schellens, J.H. A phase I and pharmacological study with imidazolium-trans-DMSO-imidazole-tetrachlororuthenate, a novel ruthenium anticancer agent. Clin. Cancer Res. 2004, 10, 3717–3727. [Google Scholar] [CrossRef]
- Brindell, M.; Stawoska, I.; Supel, J.; Skoczowski, A.; Stochel, G.; van Eldik, R. The reduction of (ImH)[trans-Ru(III)Cl4(dmso)(Im)] under physiological conditions: Preferential reaction of the reduced complex with human serum albumin. JBIC 2008, 13, 909–918. [Google Scholar] [CrossRef]
- Mahmud, K.M.; Niloy, M.S.; Shakil, M.S.; Islam, M.A. Ruthenium Complexes: An alternative to platinum drugs in colorectal cancer treatment. Pharmaceutics 2021, 13, 1295. [Google Scholar] [CrossRef]
- Monro, S.; Colon, K.L.; Yin, H.; Roque III, J.; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition metal complexes and photodynamic therapy from a tumor-centered approach: Challenges, opportunities, and highlights from the development of TLD1433. Chem. Rev. 2018, 119, 797–828. [Google Scholar] [CrossRef]
- Gichumbi, J.M.; Omondi, B.; Lazarus, G.; Singh, M.; Shaikh, N.; Chenia, H.Y.; Friedrich, H.B. Influence of halogen substitution in the ligand sphere on the antitumor and antibacterial activity of half-sandwich ruthenium(II) complexes [RuX(η6-arene)(C5H4N2-CH=N-Ar)]+. ZAAC 2017, 643, 699–711. [Google Scholar] [CrossRef]
- Zeng, L.; Gupta, P.; Chen, Y.; Wang, E.; Ji, L.; Chao, H.; Chen, Z.S. The development of anticancer ruthenium(II) complexes: From single molecule compounds to nanomaterials. Chem. Soc. Rev. 2017, 46, 5771–5804. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Samuelson, A.G. Substitution-modulated anticancer activity of half-sandwich ruthenium(II) complexes with heterocyclic ancillary ligands. Eur. J. Inorg. Chem 2014, 2014, 3536–3546. [Google Scholar] [CrossRef]
- Romero-Canelón, I.; Salassa, L.; Sadler, P.J. The contrasting activity of iodido versus chlorido ruthenium and osmium arene azo-and imino-pyridine anticancer complexes: Control of cell selectivity, cross-resistance, p53 dependence, and apoptosis pathway. J. Med. Chem. 2013, 56, 1291–1300. [Google Scholar] [CrossRef]
- Gumus, S. A computational study on substituted diazabenzenes. Turk. J. Chem. 2011, 35, 803–808. [Google Scholar] [CrossRef]
- Imran, M.; Asif, M. Study of various pyridazine and phthalazine drugs with diverse therapeutical and agrochemical activities. Russ. J. Bioorg. Chem. 2020, 46, 745–767. [Google Scholar] [CrossRef]
- Morris, R.E.; Aird, R.E.; del Socorro Murdoch, P.; Chen, H.; Cummings, J.; Hughes, N.D.; Parsons, S.; Parkin, A.; Boyd, G.; Jodrell, D.I. Inhibition of cancer cell growth by ruthenium(II) arene complexes. J. Med. Chem. 2001, 44, 3616–3621. [Google Scholar] [CrossRef]
- Gupta, G.; Prasad, K.T.; Das, B.; Yap, G.P.; Rao, K.M. Ruthenium half-sandwich complexes with tautomerized pyrazolyl-pyridazine ligands: Synthesis, spectroscopic and molecular structural studies. J. Organomet. Chem. 2009, 694, 2618–2627. [Google Scholar] [CrossRef]
- Albertin, G.; Antoniutti, S.; Castro, J.; Garcí-Fontán, S. Preparation of pyrazole-pyrazolate half-sandwich complexes of ruthenium and osmium. Eur. J. Inorg. Chem 2011, 2011, 510–520. [Google Scholar] [CrossRef]
- Gichumbi, J.M.; Friedrich, H.B.; Omondi, B. Synthesis and characterization of half-sandwich ruthenium(II) complexes with N-alkylpyridyl-imine ligands and their application in transfer hydrogenation of ketones. Transit. Metal Chem. 2016, 41, 867–877. [Google Scholar] [CrossRef]
- Gichumbi, J.M.; Friedrich, H.B.; Omondi, B. Crystal structure of chlorido-(η6–1-isopropyl-4-methyl benzene)-(1-(pyridin-2-yl)-N-(p-tolyl)methanimine-κ2N,N)ruthenium(II) hexafluorophosphate(V), C23H26ClF6N2PRu. Z. Krist. New Cryst. Struct. 2017, 232, 285–287. [Google Scholar] [CrossRef]
- Neels, A.; Stoeckli-Evans, H.; Plasseraud, L.; Fidalgo, E.G.; Süss-Fink, G. Di-μ-bromo-bis [bromo(η6-para-cymene)ruthenium(II)] benzene solvate and di-μ-iodo-bis [(η6-para-cymene)iodoruthenium(II)] toluene solvate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1999, 55, 2030–2032. [Google Scholar] [CrossRef]
- Bacchi, A.; Cantoni, G.; Pelagatti, P. Polymorphs and co-crystal with half-sandwich Ru(II) dimers [(η6-arene) RuX 2]2. CrystEngComm 2013, 15, 6722–6728. [Google Scholar] [CrossRef]
- Wang, H.Y.; Qian, Y.; Wang, F.X.; Habtemariam, A.; Mao, Z.W.; Sadler, P.J.; Liu, H.K. Ruthenium(II)–Arene Metallacycles: Crystal Structures, Interaction with DNA, and Cytotoxicity. Eur. J. Inorg. Chem 2017, 2017, 1792–1799. [Google Scholar] [CrossRef]
- Gupta, G.; Prasad, K.T.; Rao, A.V.; Geib, S.J.; Das, B.; Rao, K.M. Novel mononuclear η5-pentamethylcyclopentadienyl complexes of platinum group metals bearing pyrazolylpyridazine ligands: Syntheses and spectral studies. Inorg. Chim. Acta 2010, 363, 2287–2295. [Google Scholar] [CrossRef]
- Gichumbi, J.M.; Friedrich, H.B.; Omondi, B. Solvato-polymorph of [(η6-C6H6)RuCl (L)]PF6 (L=(2, 6-dimethyl-phenyl-pyridin-2-ylmethyleneamine). J. Mol. Struct. 2016, 1113, 55–59. [Google Scholar] [CrossRef]
- Desoize, B.; Madoulet, C. Particular aspects of platinum compounds used at present in cancer treatment. Crit. Rev. Oncol. Hematol. 2002, 42, 317–325. [Google Scholar] [CrossRef]
- Wekesa, I.M.; Jaganyi, D. Kinetic and mechanistic studies of 1, 3-bis(2-pyridylimino)isoindolatePt(II) derivatives. Experimental and new computational approach. Dalton Trans. 2014, 43, 2549–2558. [Google Scholar] [CrossRef]
- Thompson, L.K.; Woon, T.; Murphy, D.B.; Gabe, E.J.; Lee, F.L.; Le Page, Y. Binuclear copper(II) complexes of a series of tetradentate pyrazolyldiazines. Crystal and molecular structures of [μ-3,6-bis (3,5-dimethyl-1-pyrazolyl)pyridazine-N,μ-N3,μ-N3,N](μ-hydroxo)dichlorodicopper(II)aquotrichlorocuprate hydrate, Cu3C14H21Cl5N6O3, and [μ-3,6-bis (3, 5-dimethyl-1-pyrazolyl)pyridazine-N,μ-N6,μ-N7, N](μ-hydroxo)tris(nitrato)diaquodicopper(II) hydrate, Cu2C14H23N9O13. Inorg. Chem. 1985, 24, 4719–4725. [Google Scholar] [CrossRef]
- CCD CrysAlis. CrysAlis Red; Xcalibur PX Software; Oxford Diffraction Ltd.: Abingdon, UK, 2008. [Google Scholar]
- Bruker APEX2. SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Dolomanov, O.; Bourhis, L.; Gildea, R.; Howard, J.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision D. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Becke’s three parameter hybrid method using the LYP correlation functional. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).