6-Bromo-1-hydroxyhexane-1,1-bisphosphonic Acid Monosodium Salt
Abstract
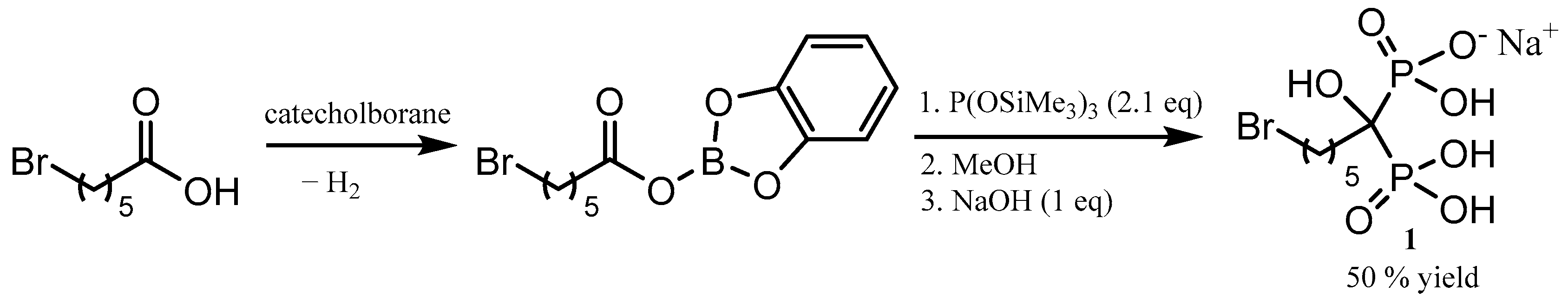
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis of 6-Bromo-1-hydroxyhexane-1,1-bisphosphonic Acid Monosodium Salt
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graham, R.; Russell, G. Bisphosphonates: The first 40 years. Bone 2011, 49, 2–19. [Google Scholar]
- Turhanen, P.A.; Demadis, K.D.; Kafarski, P. Editorial: Phosphonate Chemistry in Drug Design and Development. Front. Chem. 2021, 9, 695128. [Google Scholar] [CrossRef] [PubMed]
- Turhanen, P.A. Importance of Organophosphorus Compounds in Medicinal Chemistry Field. J. Biomed. Res. Environ. Sci. 2022, 3, 195–197. [Google Scholar]
- Matlinska, M.A.; Ha, M.; Hughton, B.; Oliynyk, A.O.; Iyer, A.K.; Bernard, G.; Lambkin, G.R.; Lawrence, M.C.; Katz, M.J.; Mar, A.; et al. Alkaline Earth Metal-Organic Frameworks with Tailorable Ion Release: A Path for Supporting Biomineralization. ACS Appl. Mater. Interfaces 2019, 11, 32739–32745. [Google Scholar] [CrossRef] [PubMed]
- Turhanen, P.A.; Vepsäläinen, J.J.; Peräniemi, S. Advanced material and approach for metal ions removal from aqueous solutions. Sci. Rep. 2015, 5, 8992. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.; Rahmani, A.; Turhanen, P.; Taskinen, A.; Nissinen, T.; Neitola, R.; Vepsäläinen, J.; Lehto, V.-P.; Riikonen, J. Recovery of uranium with bisphosphonate modified mesoporous silicon. Sep. Purif. Technol. 2021, 272, 118913. [Google Scholar] [CrossRef]
- Vassaki, M.; Papathanasiou, K.E.; Hadjicharalambous, C.; Chandrinou, D.; Turhanen, P.; Choquesillo-Lazarte, D.; Demadis, K.D. Self-Sacrificial MOFs for Ultra-Long Controlled Release of Bisphosphonate Anti-Osteoporotic Drugs. Chem. Commun. 2020, 56, 5166–5169. [Google Scholar] [CrossRef] [PubMed]
- Vassaki, M.; Kotoula, C.; Turhanen, P.; Choquesillo-Lazarte, D.; Demadis, K.D. Calcium and Strontium Coordination Polymers as Controlled Delivery Systems of the Anti-Osteoporosis Drug Risedronate and the Augmenting Effect of Solubilizers. Appl. Sci. 2021, 11, 11383. [Google Scholar] [CrossRef]
- Vassaki, M.; Lazarou, S.; Turhanen, P.; Choquesillo-Lazarte, D.; Demadis, K.D. Drug-inclusive Inorganic–Organic Hybrid Systems for the Controlled Release of the Osteoporosis Drug Zoledronate. Molecules 2022, 27, 6212. [Google Scholar] [CrossRef] [PubMed]
- Kieczykowski, G.R.; Jobson, R.B.; Melillo, D.G.; Reinhold, D.F.; Grenda, V.J.; Shinkai, I. Preparation of (4-Amino-1-Hydroxybutylidene) bisphosphonic Acid Sodium Salt, MK-217 (Alendronate Sodium). An Improved Procedure for the Preparation of 1-Hydroxy-1,1-bisphosphonic Acids. J. Org. Chem. 1995, 60, 8310–8312. [Google Scholar] [CrossRef]
- Egorov, M.; Aoun, S.; Padrines, M.; Redini, F.; Heymann, D.; Lebreton, J.; Mathé-Allainmat, M. A One-Pot Synthesis of 1-Hydroxy-1,1-bis(phosphonic acid)s Starting from the Corresponding Carboxylic Acids. Eur. J. Org. Chem. 2011, 35, 7148–7154. [Google Scholar] [CrossRef]
- Lecouvey, M.; Mallard, I.; Bailly, T.; Burgada, R.; Leroux, Y. A mild and efficient one-pot synthesis of 1-hydroxymethylene-1,1-bisphosphonic acids. Preparation of new tripod ligands. Tetrahedron Lett. 2001, 42, 8475–8478. [Google Scholar] [CrossRef]
- Nicholson, D.A.; Vaughn, H. A general method of preparation of tetramethyl alkyl-1-hydroxy-1,1-diphosphonates. J. Org. Chem. 1971, 36, 3843–3845. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turhanen, P.A. 6-Bromo-1-hydroxyhexane-1,1-bisphosphonic Acid Monosodium Salt. Molbank 2022, 2022, M1476. https://doi.org/10.3390/M1476
Turhanen PA. 6-Bromo-1-hydroxyhexane-1,1-bisphosphonic Acid Monosodium Salt. Molbank. 2022; 2022(4):M1476. https://doi.org/10.3390/M1476
Chicago/Turabian StyleTurhanen, Petri A. 2022. "6-Bromo-1-hydroxyhexane-1,1-bisphosphonic Acid Monosodium Salt" Molbank 2022, no. 4: M1476. https://doi.org/10.3390/M1476
APA StyleTurhanen, P. A. (2022). 6-Bromo-1-hydroxyhexane-1,1-bisphosphonic Acid Monosodium Salt. Molbank, 2022(4), M1476. https://doi.org/10.3390/M1476





