2-(Cyclopropylamino)-5-(4-methoxybenzylidene)thiazol-4(5H)-one
Abstract
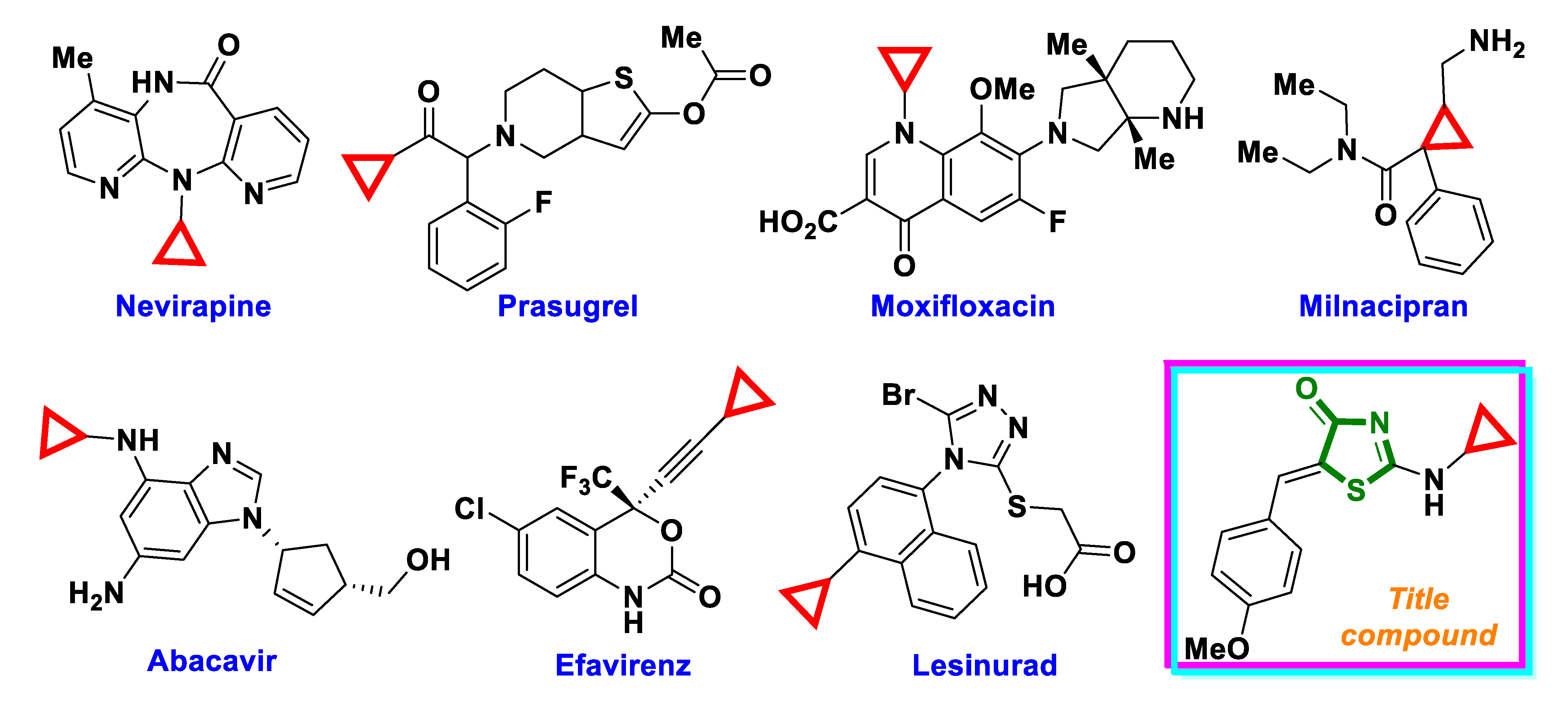
1. Introduction
2. Results and Discussion
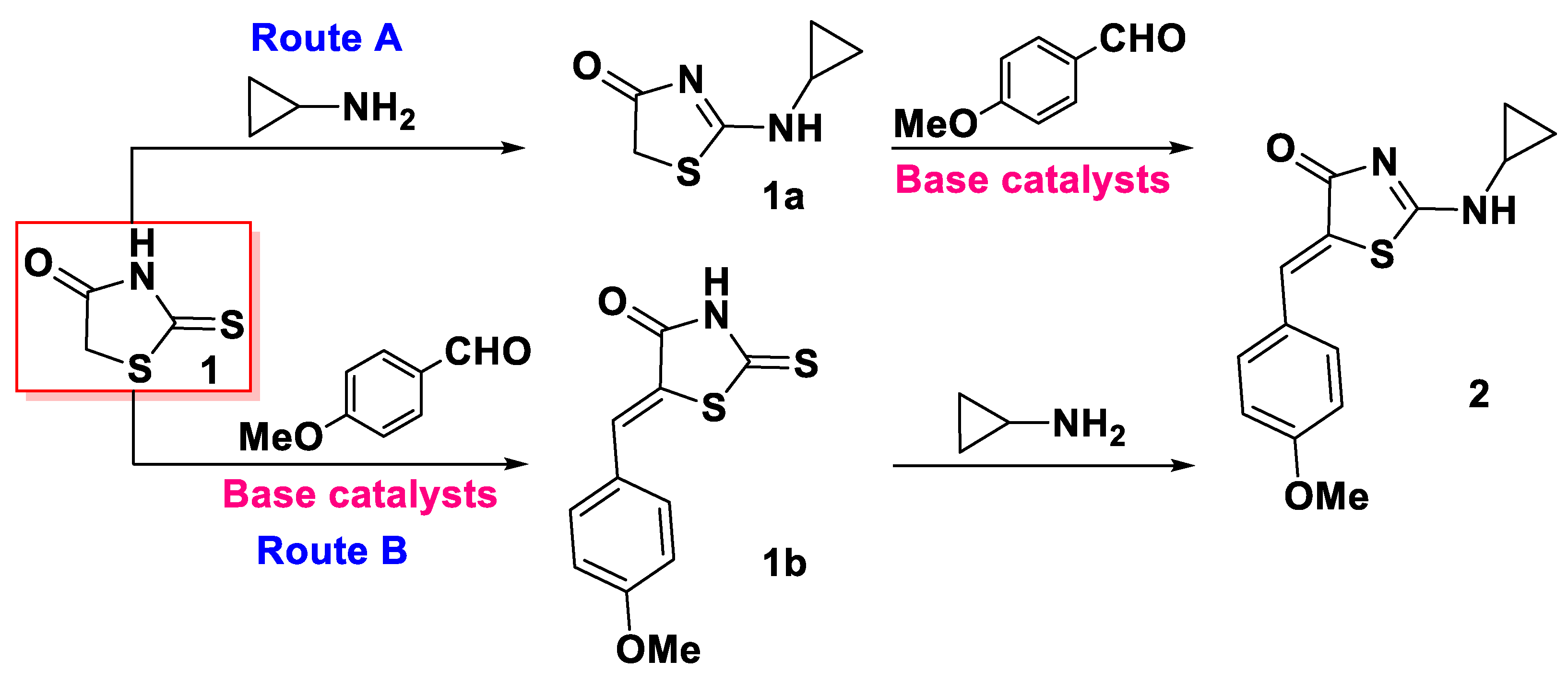
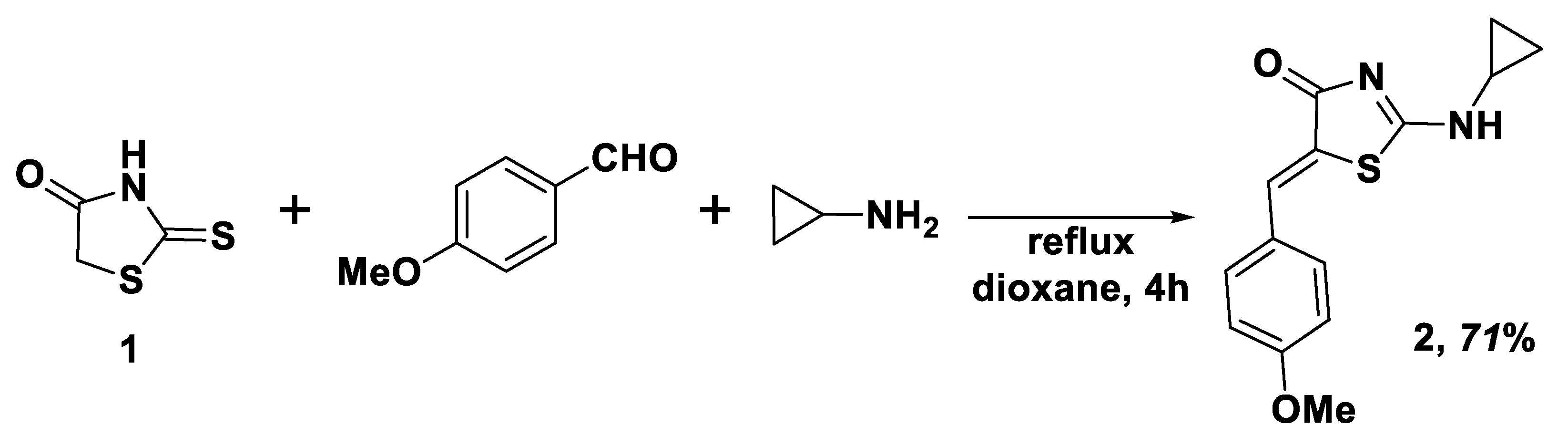
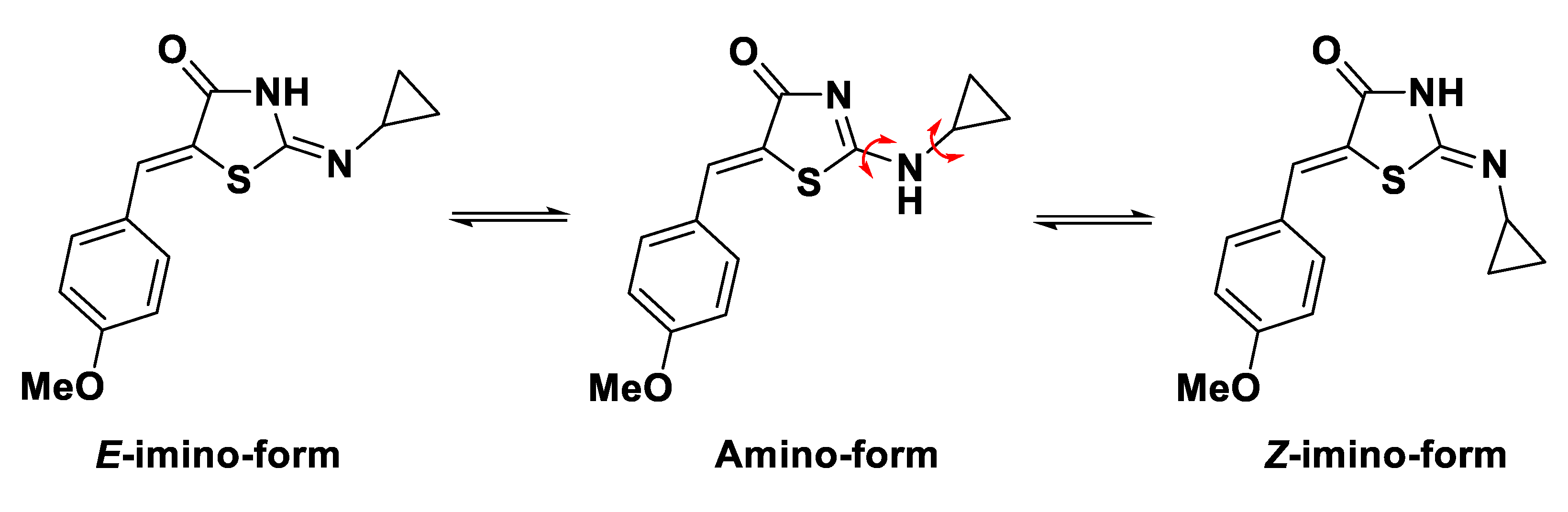
2.1. Synthesis of the Title Compound 2
2.2. Antimicrobial Activity Evaluation In Vitro of Compound 2
3. Materials and Methods
3.1. General Information and Compound 2 Synthesis
3.2. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruijter, E.; Orru, R. Synthetic and BioOrganic Chemistry Group. Multicomponent reactions in drug discovery and medicinal chemistry. Drug Discov. Today Technol. 2018, 29, 1–2. [Google Scholar] [CrossRef]
- Younus, H.A.; Al-Rashida, M.; Hameed, A.; Uroos, M.; Salar, U.; Rana, S.; Khan, K.M. Multicomponent reactions (MCR) in medicinal chemistry: A patent review (2010–2020). Expert Opin. Ther. Pat. 2021, 31, 267–289. [Google Scholar] [CrossRef] [PubMed]
- Brandão, P.; Marques, C.; Burke, A.J.; Pineiro, M. The application of isatin-based multicomponent-reactions in the quest for new bioactive and druglike molecules. Eur. J. Med. Chem. 2021, 211, 113102. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.C.; Gupta, S.J.; Fatima, G.N.; Sonar, P.K.; Verma, A.; Saraf, S.K. 4-Thiazolidinones: The advances continue…. Eur. J. Med. Chem. 2014, 72, 52–77. [Google Scholar] [CrossRef] [PubMed]
- Shepeta, Y.; Lozynskyi, A.; Sulyma, M.; Nektegayev, I.; Grellier, P.; Lesyk, R. Synthesis and biological activity evaluation of new thiazolidinone-diclofenac hybrid molecules. Phosphorus Sulfur Silicon Relat. Elem. 2020, 195, 836–841. [Google Scholar] [CrossRef]
- Ilkiv, I.I.; Lesyk, R.B.; Sklyarov, O.Ya. The influence of novel 4-thiazolidinone derivaties in cytoprotective mechanisms of small intestine under NSAID-induced damage. Ukr. Biochem. J. 2016, 88, 99–104. [Google Scholar] [CrossRef]
- Szczepański, J.; Tuszewska, H.; Trotsko, N. Anticancer Profile of Rhodanines: Structure-Activity Relationship (SAR) and Molecular Targets—A Review. Molecules 2022, 27, 3750. [Google Scholar] [CrossRef] [PubMed]
- Talele, T.T. The “cyclopropyl fragment” is a versatile player that frequently appears in preclinical/clinical drug molecules. J. Med. Chem. 2016, 59, 8712–8756. [Google Scholar] [CrossRef] [PubMed]
- Časar, Z. Synthetic approaches to contemporary drugs that contain the cyclopropyl moiety. Synthesis 2020, 52, 1315–1345. [Google Scholar] [CrossRef]
- Sun, M.R.; Li, H.L.; Ba, M.Y.; Cheng, W.; Zhu, H.L.; Duan, Y.T. Cyclopropyl Scaffold: A Generalist for Marketed Drugs. Mini-Rev. Med. Chem. 2021, 21, 150–170. [Google Scholar] [CrossRef]
- Golota, S.; Sydorenko, I.; Surma, R.; Karpenko, O.; Gzella, A.; Lesyk, R. Facile one-pot synthesis of 5-aryl/heterylidene-2-(2-hydroxyethyl-and 3-hydroxypropylamino)-thiazol-4-ones via catalytic aminolysis. Synth. Commun. 2017, 47, 1071–1076. [Google Scholar] [CrossRef]
- Holota, S.; Komykhov, S.; Sysak, S.; Gzella, A.; Cherkas, A.; Lesyk, R. Synthesis, Characterization and In Vitro Evaluation of Novel 5-Ene-thiazolo[3,2-b][1,2,4]triazole-6(5H)-ones as Possible Anticancer Agents. Molecules 2021, 26, 1162. [Google Scholar] [CrossRef] [PubMed]
- Lozynskyi, A.; Karkhut, A.; Polovkovych, S.; Karpenko, O.; Holota, S.; Gzella, A.K.; Lesyk, R. 3-Phenylpropanal and citral in the multicomponent synthesis of novel thiopyrano[2,3-d]thiazoles. Results Chem. 2022, 4, 100464. [Google Scholar] [CrossRef]
- Enchev, V.; Markova, N.; Angelov, S. Ab initio study of 2,4-substituted azolidines. II. Amino-imino tautomerism of 2-aminothiazolidine-4-one and 4-aminothiazolidine-2-one in water solution. J. Phys. Chem. A 2005, 109, 8904–8913. [Google Scholar] [CrossRef]
- Nowaczyk, A.; Kowiel, M.; Gzella, A.; Fijałkowski, L.; Horishny, V.; Lesyk, R. Conformational space and vibrational spectra of 2-[(2,4-dimethoxyphenyl)amino]-1,3-thiazolidin-4-one. J. Mol. Model. 2014, 20, 2366. [Google Scholar] [CrossRef]
- Türe, A.; Ergül, M.; Ergül, M.; Altun, A.; Küçükgüzel, İ. Design, synthesis, and anticancer activity of novel 4-thiazolidinone-phenylaminopyrimidine hybrids. Mol. Divers. 2021, 25, 1025–1050. [Google Scholar] [CrossRef]
- Nencki, M. Ueber die Einwirkung der Monochloressigsäure auf Sulfocyansäure und ihre Salze. J. Prakt. Chem. 1877, 16, 1–17. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Disk Diffusion—Manual v 10.0 (1 January 2022). Available online: https://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology/ (accessed on 5 October 2022).
| Type of Species | Species of Bacteria or Fungi | Zone of Growth Inhibition (mm ± SE) | |||||
|---|---|---|---|---|---|---|---|
| 2 | DMSO | Vancomycin | Ciprofloxacin | Clotrimazole | |||
| Gram-negative bacteria | Reference strains | Pseudomonas aeruginosa ATCC 10145 | - | - | - | 35.2 ± 0.2 | - |
| Raoultella terrigena ATCC 33257 | - | - | - | 37.3 ± 0.4 | - | ||
| Raoultella ornithinolytica DSM 7464 | 21.3 ± 0.4 | 9.2 ± 0.2 | - | 36.4 ± 0.2 | - | ||
| Clinical strains | Klebsiella pneumoniae 189 | - | - | - | 21.3 ± 0.2 | - | |
| Aeromonas hydrophila 196 | - | - | - | 20.4 ± 0.2 | - | ||
| Gram-positive bacteria | Reference strains | Streptococcus agalactiae ATCC 13813 | - | - | 24.3 ± 0.3 | 32.4 ± 0.2 | - |
| Staphylococcus aureus subsp. aureus ATCC 25923 | - | - | 25.4 ± 0.2 | 31.4 ± 0.2 | - | ||
| Staphylococcus epidermidis ATCC 12228 | - | - | 25.3 ± 0.2 | 39.5 ± 0.2 | - | ||
| Clinical strains | Enterococcus faecalis 191 | - | - | 23.2 ± 0.2 | 12.4 ± 0.2 | - | |
| Staphylococcus aureus N 23 | - | - | 11.4 ± 0.3 | 15.2 ± 0.4 | - | ||
| Fungi | Reference strain | Candidaalbicans ATCC 885–653 | 19.4 ± 0.2 | - | - | - | 19.3 ± 0.2 |
| Clinical strain | Candida albicans 67 | 28.3 ± 0.4 | 17.2 ± 0.3 | - | - | 8.4 ± 0.4 | |
| μM | ||||
|---|---|---|---|---|
| Compound 2 | Vancomycin | Ciprofloxacin | Clotrimazole | |
| Klebsiella pneumoniae 189 | 1504.3 | - | 2.2635 | - |
| Enterococcus faecalis 191 | 3008.5 | 3.4499 | 12.0722 | - |
| Candida albicans 67 | 752.1285 | - | - | 5.8 |
| Raoultella ornithinolytica DSM 7464 | 752.1285 | - | 0.754512 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sydorenko, I.; Holota, S.; Lozynskyi, A.; Konechnyi, Y.; Horishny, V.; Karkhut, A.; Polovkovych, S.; Karpenko, O.; Lesyk, R. 2-(Cyclopropylamino)-5-(4-methoxybenzylidene)thiazol-4(5H)-one. Molbank 2022, 2022, M1478. https://doi.org/10.3390/M1478
Sydorenko I, Holota S, Lozynskyi A, Konechnyi Y, Horishny V, Karkhut A, Polovkovych S, Karpenko O, Lesyk R. 2-(Cyclopropylamino)-5-(4-methoxybenzylidene)thiazol-4(5H)-one. Molbank. 2022; 2022(4):M1478. https://doi.org/10.3390/M1478
Chicago/Turabian StyleSydorenko, Ivan, Serhii Holota, Andrii Lozynskyi, Yulian Konechnyi, Volodymyr Horishny, Andriy Karkhut, Svyatoslav Polovkovych, Olexandr Karpenko, and Roman Lesyk. 2022. "2-(Cyclopropylamino)-5-(4-methoxybenzylidene)thiazol-4(5H)-one" Molbank 2022, no. 4: M1478. https://doi.org/10.3390/M1478
APA StyleSydorenko, I., Holota, S., Lozynskyi, A., Konechnyi, Y., Horishny, V., Karkhut, A., Polovkovych, S., Karpenko, O., & Lesyk, R. (2022). 2-(Cyclopropylamino)-5-(4-methoxybenzylidene)thiazol-4(5H)-one. Molbank, 2022(4), M1478. https://doi.org/10.3390/M1478










