Abstract
2-Cyano-3-(1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)acrylamide (3) was synthesized in 90% yield from condensation of equimolar equivalents of 1-phenyl-3-(thiophen-2-yl)-1H-pyrazole-4-carbaldehyde (1) and 2-cyanoacetamide (2) in boiling ethanol under basic condition for 45 min. The structure of 3 was determined using NMR spectroscopy and single crystal X-ray diffraction.
1. Introduction
Compounds containing the acrylamide residue are potentially biologically active as well as acting as precursors in many organic syntheses [1,2,3,4]. Pyrazoles are also important heterocycles with promise for medicinal applications as a result of their wide range of biological activities [5,6]. In addition, heterocycles containing thiophene ring systems have many latent applications [7,8,9]. The synthesis of heterocycles containing both pyrazole and thiophene moieties is therefore an intriguing prospect.
The most recent synthetic procedures for the production of pyrazoles involve cycloaddition of N-isocyanoiminotriphenylphosphorane and terminal alkynes in the presence of a catalyst [10], one-pot condensation of carbonyl compounds and hydrazine monohydrochloride in the presence of oxygen [11], cyclization of β,γ-unsaturated hydrazones in the presence of oxygen and a copper catalyst [12], and the reaction of diarylhydrazones and vicinal diols in the presence of iron-containing catalyst [13]. In the case of thiophene derivatives, synthesis involves Paal–Knorr reaction [14,15], Gewald reaction [16,17,18], and reactions involving sulfuration and cyclization processes of alkynes [19,20,21]. It has been reported that 2-cyanoacrylamides can be used as an active ingredient in chemotherapy [22]. In this paper, we report the synthesis and characterization of 2-cyano-3-(1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)acrylamide (3). The structures of several related compounds that contain the pyrazole and thiophene ring systems have been reported [23,24,25,26,27].
2. Results and Discussion
2.1. Synthesis of 3
The condensation of equimolar equivalents of 1-phenyl-3-(thiophen-2-yl)-1H-pyrazole-4-carbaldehyde (1) and 2-cyanoacetamide (2) in boiling ethanol under basic conditions for 45 min gave 2-cyano-3-(1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)acrylamide (3) in 90% yield (Scheme 1). The structure of 3 was determined using NMR spectroscopy (See Section 3.2. for details) and confirmed by single crystal X-ray diffraction (Figure 1).
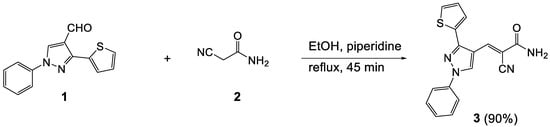
Scheme 1.
Synthesis of 3.
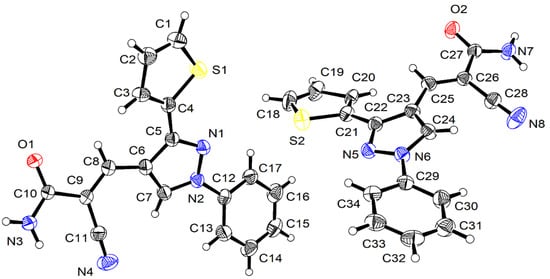
Figure 1.
Ortep representation of the asymmetric unit of 3 showing 50% probability atomic displacement parameters.
2.2. NMR Spectroscopy
The 1H NMR spectrum of 3 showed the presence of 12 protons with the NH2 protons appearing as an exchangeable singlet at low field (δ = 9.11 ppm). The pyrazole proton appears as a singlet at 8.16 ppm. The 1H NMR spectrum showed the presence of a doublet (two protons) and two triplets (one and two protons) corresponding to the five protons of the phenyl ring. The 13C NMR spectrum of 3 showed the expected signals for all carbons with the carbonyl (C=O) and nitrile (C≡N) carbons appearing at 163.0 and 117.3 ppm, respectively. The NMR spectra for 3 are included in the supplementary materials.
2.3. X-ray Structure
The asymmetric unit of the crystal structure consists of two independent molecules. The molecules comprise thiophenyl ((A1: C1–C4, S1) and (A2: C18–C21, S2)), pyrazolyl ((B1: C5–C7, N1, N2) and (B2: C22–C24, N5, N6)), and phenyl ((D1: C12–C17) and (D2: C29–C34)) rings and cyanoacrylamide ((C1: C8–C11, N3, N4, O1) and (C2: C25–C28, N7, N8, O2)) groups.
The conformations of the two independent molecules are very similar in the crystal, as shown by the twist angles between adjacent groups. The angles between the groups are: A1/B1 = 18.9 (2)°, B1/C1 = 24.8 (2)°, and B1/D1 = 24.5 (2)° for the first molecule and A2/B2 = 23.6 (2)°, B2/C2 = 24.2 (2)°, and B2/D2 = 26.5 (2)° for the second molecule.
In the crystal structure (Figure 2), independent pairs of molecules are linked by two N–H···O hydrogen bonds (with geometry N3···O2 = 2.898 (4)Å, N3-H3B···O2 = 168.8° and N7···O1 = 2.886 (4)Å, N7–H7A···O1 = 167.9°) to form R(8)22 rings. Additional bonds of type N-H···N (with geometry N3···N8 = 3.079 (5)Å, N3–H3C···N8 = 150.6°, and N7···N4 = 3.107 (5)Å, N7–H7B···N4 = 149.4°) lead to the formation of molecular chains through the structure parallel to [100].
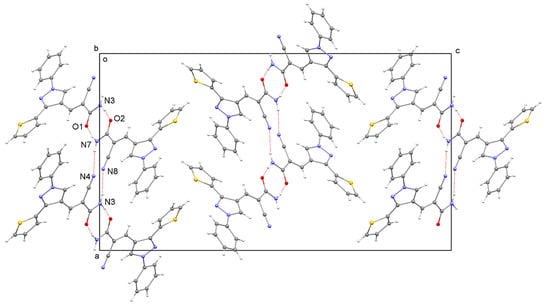
Figure 2.
Crystal structure packing viewed down the b axis with hydrogen bonding contacts shown as red dashed lines.
3. Materials and Methods
3.1. General
The melting point of 3 was determined using an Electrothermal melting point apparatus. The IR spectrum of 3 was recorded on a JASCO FT/IR-4600 spectrometer. The NMR spectra of 3 were measured on a JEOLNMR 500 MHz spectrometer at 500 MHz for the 1H and 125 MHz for the 13C NMR. The coupling constant (J) and the chemical shift (δ) are reported in Hz and ppm, respectively. Compound 1 was prepared based on a literature procedure [28].
3.2. Synthesis of 3
A mixture of 1 (0.51 g, 2.0 mmol) and 2 (0.17 g, 2.0 mmol) in ethanol (10 mL) containing piperidine (0.17 g, 2.0 mmol) was refluxed for 45 min. The mixture was cooled to room temperature and the solid formed was filtered, washed with ethanol, and dried. The crude product was recrystallized from dimethylformamide to give pale yellow crystals of 3. Yield: 90%, Mp: 248–250 °C. IR (KBr): 3599 (NH2), 3375 (NH2), 3143 (CH), 2796 (CH), 2225 3 (C≡N), 1697 (C=O), 1589 (C=N), 1381 (C–C), 1227 (C–O) cm−1. 1H NMR (DMSO-d6): 7.24 (t, J = 7.7 Hz, 1H, Ph), 7.42–7.46 (m, 2H, thienyl), 7.57 (t, J = 7.7 Hz, 2H, Ph), 7.74 (br, 1H, thienyl), 7.87 (d, J = 7.7 Hz, 2H, Ph), 7.95 (s, 1H, olefin), 8.16 (s, 1H, pyrazolyl), 9.11 (s, exch., 2H, NH2). 13C NMR (DMSO-d6): 105.7 (C–C≡N), 114.7 (C4 of pyrazolyl), 117.3 (C≡N), 120.0 (C2/C6 of Ph), 128.6 (C4 of thienyl), 128.66 (C4 of thienyl), 128.69 (C4 of Ph), 128.9 (C5 of thienyl), 129.9 (C5 of pyrazolyl), 130.4 (C3/C5 of Ph), 132.7 (C3 of pyrazolyl), 138.9 (C1 of Ph), 141.4 (C2 of thienyl), 148.9 (CH), 163.0 (C=O).
3.3. Data Collection and Refinement Details
An Agilent SuperNova Dual Atlas diffractometer using mirror monochromated CuKα radiation (λ = 1.54184 Å) was used to collect single crystal diffraction data. The structure of 3 was solved by direct methods using SHELXS [29] and refined by full-matrix least-squares methods on F2 with SHELXL-2014 [30]. Crystal Data: C17H12N4OS (M = 320.37 g/mol), Orthorhombic, space group Pca21, 0.32 × 0.14 × 0.04 mm, a = 17.2514 (3) Å, b = 5.8392 (1) Å, c = 30.8194 (6) Å, V = 3104.57 (10) Å3, Z = 8, T = 296 K, µ(Cu Kα) = 1.93 mm−1, Dcalc = 1.371 Mg m−3, 10,454 reflections measured (θ = 5.1–72.6°), 5046 unique, Rint = 0.024, R1 = 0.0432, wR2 = 0.1209 for I > 2σ(I)] and R1 = 0.0451, wR2 = 0.1242 for all data. The X-ray crystallographic data for compound 3 have been deposited in the Cambridge Crystallographic Data Center with CCDC reference number 2169792.
4. Conclusions
2-Cyano-3-(1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)acrylamide was synthesized in excellent yield using a simple procedure and its structure was established based on the data NMR spectroscopy and single crystal X-ray diffraction analysis.
Supplementary Materials
The following are available online, IR, 1H, and 13C NMR spectra, CIFs and check if reports for the title compound.
Author Contributions
Conceptualization: B.M.K. and G.A.E.-H.; methodology: B.M.K., B.F.A.-W., H.A.M. and G.A.E.-H.; X-ray crystal structures: B.M.K.; investigation: B.M.K., B.F.A.-W., H.A.M. and G.A.E.-H.; writing—original draft preparation: B.M.K., B.F.A.-W., H.A.M. and G.A.E.-H.; writing—review and editing: B.M.K. and G.A.E.-H. All authors have read and agreed to the published version of the manuscript.
Funding
G.A.E.-H. is grateful to the Deanship of Scientific Research, King Saud University for funding through Vice Deanship of Scientific Research Chairs.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and the supplementary material.
Acknowledgments
We thank Cardiff University and National Research Centre for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ren, Z.-L.; Liu, H.; Jiao, D.; Hu, H.-T.; Wang, W.; Gong, J.-X.; Wang, A.-L.; Cao, H.-Q.; Lv, X.-H. Design, synthesis, and antifungal activity of novel cinnamon-pyrazole carboxamide derivatives. Drug Dev. Res. 2018, 79, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ni, Y.; Chen, J.; Tu, Z.; Wu, X.; Chen, D.; Yao, H.; Jiang, S. Discovery of trans-3-(pyridin-3-yl)acrylamide-derived sulfamides as potent nicotinamide phosphoribosyltransferase (NAMPT) inhibitors for the potential treatment of cancer. Bioorg. Med. Chem. Lett. 2019, 29, 1502–1506. [Google Scholar] [CrossRef] [PubMed]
- Alafeefy, A.M.; Isik, S.; Abdel-Aziz, H.A.; Ashour, A.E.; Vullo, D.; Al-Jaber, N.A.; Supuran, C.T. Carbonic anhydrase inhibitors: Benzenesulfonamides incorporating cyanoacrylamide moieties are low nanomolar/subnanomolar inhibitors of the tumor-associated isoforms IX and XII. Bioorg. Med. Chem. 2013, 21, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, B.F.; Farahat, A.A.; Awad, E.A.; El-Hiti, G.A. Synthesis and antimicrobial activity of some novel substituted 3-(thiophen-2-yl)pyrazole-based heterocycles. Lett. Drug Design Discov. 2017, 14, 1316–1323. [Google Scholar] [CrossRef]
- Faria, J.V.; Vegi, P.F.; Miguita, A.G.C.; dos Santos, M.S.; Boechat, N.; Bernardino, A.M.R. Recently reported biological activities of pyrazole compounds. Bioorg. Med. Chem. 2017, 21, 5891–5903. [Google Scholar] [CrossRef]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-Aizari, F.A.; Ansar, M. Synthesis and pharmacological activities of pyrazole derivatives: A review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef] [Green Version]
- Kanwal, I.; Rasool, N.; Zaidi, S.H.M.; Zakaria, Z.A.; Bilal, M.; Hashmi, M.A.; Mubarik, A.; Ahmad, G.; Shah, S.A.A. Synthesis of functionalized thiophene based pyrazole amides via various catalytic approaches: Structural features through computational applications and nonlinear optical properties. Molecules 2022, 27, 360. [Google Scholar] [CrossRef]
- Prabhudevaa, M.G.; Renukab, N.; Kumar, K.A. Synthesis of thiophene-pyrazole conjugates as potent antimicrobial and radical scavengers. Curr. Chem. Lett. 2018, 7, 73–80. [Google Scholar] [CrossRef]
- da Cruz, R.M.D.; Mendonça-Junior, F.J.B.; de Mélo, N.B.; Scotti, L.; de Araújo, R.S.A.; de Almeida, R.N.; de Moura, R.O. Thiophene-based compounds with potential anti-inflammatory activity. Pharmaceuticals 2021, 14, 692. [Google Scholar] [CrossRef]
- Yi, F.; Zhao, W.; Wang, Z.; Bi, X. Silver-mediated [3 + 2] cycloaddition of alkynes and N-isocyanoiminotriphenylphosphorane: Access to monosubstituted pyrazoles. Org. Lett. 2019, 21, 3158–3161. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Jia, X. Phosphine-free [3+2] cycloaddition of propargylamines with dialkyl azodicarboxylates: An efficient access to pyrazole backbone. Synthesis 2018, 50, 3499–3505. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.; Feng, J.; Hou, Y.; Rao, M.; Cheng, J. Copper-catalyzed aerobic cyclization of β,γ-unsaturated hydrazones with concomitant C=C bond cleavage. Org. Lett. 2020, 22, 7981–7985. [Google Scholar] [CrossRef] [PubMed]
- Panda, N.; Jena, A.K. Fe-catalyzed one-pot synthesis of 1,3-di- and 1,3,5-trisubstituted pyrazoles from hydrazones and vicinal diols. J. Org. Chem. 2012, 77, 9401–9406. [Google Scholar] [CrossRef] [PubMed]
- Minetto, G.; Raveglia, L.F.; Sega, A.; Taddei, M. Microwave-assisted Paal–Knorr reaction—Three-step regiocontrolled synthesis of polysubstituted furans, pyrroles and thiophenes. Eur. J. Org. Chem. 2005, 2005, 5277–5288. [Google Scholar] [CrossRef]
- Kaleta, Z.; Makowski, B.T.; Soos, T.; Dembinski, R. Thionation using fluorous Lawesson’s reagent. Org. Lett. 2006, 8, 1625–1628. [Google Scholar] [CrossRef]
- Ma, L.; Yuan, L.; Xu, C.; Li, G.; Tao, M.; Zhang, W. An efficient synthesis of 2-aminothiophenes via the Gewald reaction catalyzed by an N-methylpiperazine-functionalized polyacrylonitrile fiber. Synthesis 2013, 45, 45–52. [Google Scholar] [CrossRef]
- Revelant, G.; Dunand, S.; Hesse, S.; Kirsch, G. Microwave-assisted synthesis of 5-substituted 2-aminothiophenes starting from arylacetaldehydes. Synthesis 2011, 2011, 2935–2940. [Google Scholar] [CrossRef]
- Wang, T.; Huang, X.-G.; Liu, J.; Li, B.; Wu, J.-J.; Chen, K.-X.; Zhu, W.-L.; Xu, X.-Y.; Zeng, B.-B. An Efficient one-pot synthesis of substituted 2-aminothiophenes via three-component Gewald reaction catalyzed by L-proline. Synlett 2010, 2010, 1351–1354. [Google Scholar] [CrossRef]
- Huang, G.; Li, J.; Li, J.; Li, J.; Sun, M.; Zhou, P.; Chen, L.; Huang, Y.; Jiang, S.; Li, Y. Access to substituted thiophenes through xanthate-mediated vinyl C(sp2)-Br bond cleavage and heterocyclization of bromoenynes. J. Org. Chem. 2020, 85, 13037–13049. [Google Scholar] [CrossRef]
- Gabriele, B.; Mancuso, R.; Veltri, L.; Maltese, V.; Salerno, G. Synthesis of substituted thiophenes by palladium-catalyzed heterocyclodehydration of 1-mercapto-3-yn-2-ols in conventional and nonconventional solvents. J. Org. Chem. 2012, 77, 9905–9909. [Google Scholar] [CrossRef]
- Zhang, G.; Yi, H.; Chen, H.; Bian, C.; Liu, C.; Lei, A. Trisulfur radical anion as the key intermediate for the synthesis of thiophene via the interaction between elemental sulfur and NaOtBu. Org. Lett. 2014, 16, 6156–6159. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.F.; Saddiq, A.A.; Abdelhamid, I.A. Attacking the mitochondria of colorectal carcinoma by novel 2-cyanoacrylamides linked to ethyl 1,3-diphenylpyrazole-4-carboxylates moiety as a new trend for chemotherapy. Bioorg. Chem. 2020, 103, 104195. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, A.A.; Abdel-Wahab, B.F.; Hegazy, A.S.; Kariuki, B.M.; El-Hiti, G.A. The crystal structure of 5-(2-(4-fluorophenyl)hydrazono)-4-methyl-2-((3-(5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)hydrazono)-2,5-dihydrothiazole dimethylformamide monosolvate, C30H25FN10S.C3H7NO. Z. Kristallogr.-New Cryst. Struct. 2020, 235, 915–917. [Google Scholar] [CrossRef]
- Alotaibi, A.A.; Abdel-Wahab, B.F.; Hegazy, A.S.; Kariuki, B.M.; El-Hiti, G.A. The crystal structure of 2-(3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-8H-indeno[1,2-d]thiazole, C25H17BrFN3S. Z. Kristallogr.-New Cryst. Struct. 2020, 235, 897–899. [Google Scholar] [CrossRef]
- Alotibi, M.F.; Abdel-Wahab, B.F.; Yousif, E.; Hegazy, A.S.; Kariuki, B.M.; El-Hiti, G.A. Crystal structure of (E)-3-(3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1-phenylprop-2-en-1-one, C27H21N5O. Z. Kristallogr.-New Cryst. Struct. 2020, 235, 479–481. [Google Scholar] [CrossRef] [Green Version]
- Baashen, M.A.; Abdel-Wahab, B.F.; Hegazy, A.S.; Kariuki, B.M.; El-Hiti, G.A. The crystal structure of 1-phenyl-N-(4,5,6,7-tetrabromo-1,3-dioxoisoindolin-2-yl)-5-(thiophen-2-yl)-1H-pyrazole-3-carboxamide-dimethylformamdie (1/1) C22H10Br4N4O3S. Z. Kristallogr.-New Cryst. Struct. 2021, 236, 431–433. [Google Scholar] [CrossRef]
- El-Hiti, G.A.; Abdel-Wahab, B.F.; Baashen, M.; Ghabbour, H.A. Crystal structure of ethyl 5-amino-3-(methylthio)-1-(1-phenyl-5-(thiophen-2-yl)-1H-pyrazole-3-carbonyl)-1H-pyrazole-4-carboxylate, C21H19N5O3S2. Z. Kristallogr.-New Cryst. Struct. 2016, 231, 1051–1052. [Google Scholar] [CrossRef] [Green Version]
- Bratenko, M.K.; Sidorchuk, I.I.; Khalaturnik, M.V.; Vovk, M.V. Synthesis and antimicrobial activity of new azomethines synthesized from 4-formyl-1-phenyl-3-aryl(heteryl)pyrazoles. Pharm. Chem. J. 1999, 33, 81–83. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).