Abstract
This short note elaborates a concise protocol for the synthesis of two novel vicinal haloethers bearing a malonontrile group, 2-bromo-2-(methoxy(phenyl)methyl)malononitrile (1) and 2-iodo-2-(methoxy(phenyl)methyl)malononitrile (2). The structures of the synthesized compounds were confirmed by 1H, 13C-NMR spectroscopy. The final products indicate that methanol not only acts as solvent but also participates in and dominates the reaction result.
1. Introduction
The difunctionalization of olefins has become an attractive strategy for rapidly increasing molecular complexity from abundant and cheap feedstock [1,2,3,4]. Up to now, much work on difunctionlization has been reported, such as the representative aminohalogenation reaction, which provides an effective platform to convert simple olefins [5,6,7], α, β-unsaturated ketones [8,9,10], and β-nitrostyrene derivatives [11,12,13] into vicinal haloamine compounds. β, β-Dicyanostyrene derivatives are a kind of reaction raw material containing a multifunctional group which can be converted into the skeleton structures in drugs and natural products, via difunctionalization, such as enamine [14], β-hydroxy sulfide [15], dicyanocyclobutane [16], and so on. Recently, an unexpected vicinal bromoether product was obtained when we performed an aminobromination reaction of β, β-dicyanostyrene. On this basis, we developed a synthetic protocol for vicinal haloethers, including 2-bromo-2-(methoxy(phenyl)methyl)malononitrile (1) and 2-iodo-2-(methoxy(phenyl)methyl)malononitrile (2), which are verified to be novel compounds and to possess the potential to be building blocks in organic synthesis by easy conversion into amounts of useful functional derivatives via intermolecular or intramolecular nucleophilic substitution of halogens.
2. Results
Many synthetic methods are available for the aminobromination reaction of β, β-dicyanostyrene; most of them require catalysts such as Na2CO3 [14], K3PO4 [17], and NaHCO3 [18]. In 2012, Chen reported a NaOAc-catalyzed aminobromination of β, β-dicyanostyrene, with NBS as nitrogen/bromine source, to yield N-[2-Bromo-2,2-dicyano-1-phenylethyl]pyrrolidine-2,5-dione [19]. Recently, we slightly modified this reaction by replacing CH3CN with methanol as the solvent and abandoning the catalyst. Surprisingly, the expected product was not observed in the reaction. Alternatively, β, β-dicyanostyrene was converted into 2-bromo-2-(methoxy(phenyl)methyl)malononitrile (1) in moderate yield (Scheme 1). Subsequently, we also tried ethanol, isopropyl alcohol, and tert-butanol as solvents, but none of the reactions formed 2-bromo-2-(methoxy (phenyl)methyl)malononitrile or N-[2-bromo-2,2-dicyano-1-phenylethyl]pyrrolidine-2,5-dione. Most of the starting material, β, β-dicyanostyrene, was recovered, and some was converted to benzaldehyde. The relative mechanism is still under further study.
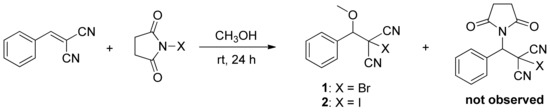
Scheme 1.
Synthesis of compound 1 and 2.
After optimization of the experimental conditions (details of the condition optimization are shown in Table S1), the reaction was performed in methanol at room temperature by using β, β-dicyanostyrene/NBS (1:1 ratio) as the starting materials. Compound 1 was obtained in 76.8% yield and confirmed by NMR. Firstly, the 1H-NMR spectrum (Supplementary Materials) exhibits the typical signals for the methoxy and methyne group as a single peak at 3.47 and 4.67 ppm, respectively. The chemical shifts of the five hydrogen atoms on the substituted benzene ring are concentrated in the range of 7.45 to 7.55 ppm. The 13C-NMR spectrum exhibits nine peaks which fully agree with the proposed structure for 1 (Supplementary Materials).
As an expansion of the reaction, N-iodosuccinimide (NIS) was selected as the iodine source, and we obtained 2-iodo-2-(methoxy(phenyl)methyl)malononitrile (2) in 70.5% yield under the same reaction conditions. The molecular structure of compound 2 was also unambiguously confirmed by 1H-NMR and 13C-NMR, with the peak at 1.02 ppm in the 13C-NMR spectrum representing the typical chemical shift of C–I bond.
3. Discussion
All reagents were purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China) and used without further purification. The starting β, β-dicyanostyrene was synthesized according to the literature [17]. NMR spectra were recorded on a Bruker Avance AV400 (400/100 MHz 1H/13C) spectrometer (Bruker, Billerica, MA, USA), and chemical shifts (δ, ppm) were down field from TMS.
2-Bromo-2-(methoxy(phenyl)methyl)malononitrile (1): in a 100 mL three-neck flask, N-bromosuccinimide (0.35 g, 2 mmol) was added to the solution of β, β-dicyanostyrene (0.31 g; 2 mmol) in absolute methanol (10 mL). The reaction mixture was stirred at room temperature for 24 h, and then the excess methanol was removed by rotary evaporation. The residue was purified by column chromatography over silica gel (EtOAc/petroleum = 1/10) to yield a yellow oil (0.34 g, 76.8%). 1H NMR (400 MHz, CDCl3): δ 7.55 (dd, J = 7.4, 2.1 Hz, 2H), 7.52–7.45 (m, 3H), 4.64 (s, 1H), 3.47 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 131.38 (s), 130.94 (s), 128.96 (s), 128.60 (s), 111.11 (s), 110.81 (s), 86.05 (s), 58.74 (s), 30.54 (s).
2-Iodo-2-(methoxy(phenyl)methyl)malononitrile (2): in a 100 mL three-neck flask, N-iodosuccinimide (0.45 g, 2 mmol) was added to the solution of β, β-dicyanostyrene (0.31 g; 2 mmol) in absolute methanol (10 mL). The reaction mixture was stirred at room temperature for 24 h, and then the excess methanol was removed by rotary evaporation. The residue was purified by column chromatography over silica gel (EtOAc/petroleum = 1/10) to yield a brown oil (0.44 g, 70.5%). 1H NMR (400 MHz, CDCl3) δ 7.58–7.54 (m, 2H), 7.53–7.48 (m, 3H), 4.45 (s, 1H), 3.48 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 131.83 (s), 130.80 (s), 129.00 (s), 128.38 (s), 112.87 (s), 112.66 (s), 86.21 (s), 58.61 (s).
4. Conclusions
Two novel vicinal haloether compounds, 2-bromo-2-(methoxy(phenyl)methyl)malononitrile (1) and 2-iodo-2-(methoxy(phenyl)methyl)malononitrile (2), were handily synthesized from β, β-dicyanostyrene and characterized by NMR. Future work will emphasize exploring the scope of β, β-dicyanostyrene derivatives and illustrating the reaction mechanism.
Supplementary Materials
The following supporting information can be downloaded online, 1H-NMR and 13C-NMR spectra of compounds 1, 2. Reference [17] is cited in Supplementary Materials. Table S1. condition optimization. Figure S1. H-NMR and 13C-NMR spectra of compound 1. Figure S2. 1H NMR and 13C NMR spectra of compound 2.
Author Contributions
Investigation, J.C. and L.D.; writing—original draft preparation, K.L.; writing—review and editing, J.-B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Jiangxi Provincial Natural Science Foundation (20202BABL213007, 20212BAB203013), National College Students’ Innovation and Entrepreneurship Training Program (202110407006). The Youth Jinggang Scholars Program in Jiangxi Province is gratefully acknowledged.
Data Availability Statement
The data from this study are available in this paper and in its Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhong, L.-J.; Xiong, Z.-Q.; Ouyang, X.-H.; Li, Y.; Song, R.-J.; Sun, Q.; Lu, X.; Li, J.-H. Intermolecular 1,2-difunctionalization of alkenes enabled by fluoroamide-directed remote benzyl C(sp3)-H functionalization. J. Am. Chem. Soc. 2022, 144, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Bao, Y.Y.; Tang, M.F.; Ye, Z.G.; Yuan, Z.L.; Zhu, G.G. Recent advances in difunctionalization of alkenes using pyridinium salts as radical precursors. Chem. Commun. 2022, 58, 3847–3864. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Wu, D.; Cheng, H.-G.; Yin, G.Y. Difunctionalization of alkenes involving metal migration. Angew. Chem. Int. Ed. 2020, 5, 7990–8003. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.-H.; Hua, Y.H.; Lin, Y.-M.; Fei, J.W.; Gao, L.-H.; Zhao, X.D.; Xia, H.P. Selective difunctionalization of unactivated aliphatic alkenes enabled by a metal-metallaaromatic catalytic system. J. Am. Chem. Soc. 2022, 144, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.V.; Talluri, S.K.; Sudalai, A. Transition metal-catalyzed regio- and stereoselective aminobromination of olefins with TsNH2 and NBS as nitrogen and bromine Sources. Org. Lett. 2003, 5, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-L.; Wang, G.-W. Aminohalogenation of electron-deficient olefins promoted by hypervalent iodine compounds. Eur. J. Org. Chem. 2008, 2008, 6239–6246. [Google Scholar] [CrossRef]
- Bovino, M.T.; Chemler, S.R. Catalytic enantioselective alkene aminohalogenation/cyclization involving atom transfer. Angew. Chem. Int. Ed. 2012, 51, 3923–3927. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-G.; Wei, J.-F.; Wang, M.-Z.; Zhou, L.-Y.; Zhang, C.-J.; Shi, X.-Y. Aluminium powder-catalyzed regio- and stereoselective aminobromination of α,β-unsaturated carbonyl compounds and simple olefins with the p-toluenesulfonamide/N-bromosuccinimide (TsNH2-NBS) system. Adv. Synth. Catal. 2009, 351, 2358–2368. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Li, G. Regio- and stereoselective synthesis of anti-1,3-Diaryl-3-chloro-2-(o-nitrophenylsulfonylamino)-3-propan-1-ones through catalytic aminohalogenation reaction of α,β-Unsaturated Ketones. Eur. J. Org.Chem. 2006, 2006, 3112–3115. [Google Scholar] [CrossRef]
- Chen, Z.-G.; Wei, J.-F.; Li, W.-L.; Wang, Y.; Zhao, P.-F.; Shi, X.-Y. (+)-Tartaric acid-catalyzed high regio- and stereoselective aminobromination of olefins. Chin. J. Chem. 2011, 29, 1689–1696. [Google Scholar] [CrossRef]
- Zhi, S.J.; Sun, H.; Lin, C.; Zhang, G.Q.; Li, G.G.; Pan, Y. Regioselective aminohalogenation of β-nitrostyrenes using NCS and NBS as nitrogen/halogen sources. Sci. China Chem. 2010, 53, 140–146. [Google Scholar] [CrossRef]
- Mei, H.B.; Han, J.L.; Lia, G.G.; Pan, Y. KOH-catalyzed highly efficient aminohalogenation of β-nitrostyrenes with t-butyl N,N-dichlorocarbamate as nitrogen/halogen source. RSC Adv. 2011, 1, 429–433. [Google Scholar] [CrossRef]
- Zhi, S.-J.; Sun, H.; Zhang, G.-Q.; Li, G.; Pan, Y. New catalytic system for aminohalogenation of β-methyl-β-nitrostyrenes to give opposite regiochemistry. Org. Biomol. Chem. 2010, 8, 628–631. [Google Scholar] [CrossRef]
- Kang, N.; Chen, Z.G. Base-catalyzed formation of enamines from β,β-dicyanostyrene derivatives with N-bromosaccharin. Chem. Res. Chin. Univ. 2018, 34, 751–757. [Google Scholar] [CrossRef]
- Azizi, B.; Heravi, M.R.P.; Hossaini, Z.; Ebadid, A.; Vessally, A. Intermolecular difunctionalization of alkenes: Synthesis of β-hydroxy sulfides. RSC Adv. 2021, 11, 13138–13151. [Google Scholar] [CrossRef] [PubMed]
- Scheeren, H.W.; Rossun Van, A.J.R.; Nivard, R.J.F. The influence of the H-electron distribution and H-bond stability of ketene acetals on their reactivity and stereoselectivity in thermal (2+2) cycloadditions with 1,1-dicyanostyrenes. Tetrahedron 1983, 39, 1345–1353. [Google Scholar] [CrossRef]
- Chen, Z.G.; Li, Y.N.; Zhou, J.M.; Wang, D.; Miao, G. K3PO4-catalyzed regiospecific bromoamidation of β,β-dicyanostyrene derivatives with N-bromoacetamide (NBA). Chem. Res. Chin. Univ. 2014, 30, 266–271. [Google Scholar] [CrossRef]
- Chen, Z.G.; Xia, W.; Liu, D.E.; Liu, Y.L.; Du, M.F.; Cao, C.X. NaHCO3-catalyzed highly regiospecific aminobromination of β,β-dicyanostyrene derivatives with 1,3-Dibromo-5,5-dimethylhydantoin (DBDMH). J. Chin. Chem. Soc. 2016, 63, 158–164. [Google Scholar] [CrossRef]
- Li, W.L.; Chen, Z.G.; Zhou, J.M.; Hu, J.L.; Xia, W. Sodium acetate-catalyzed regiospecific and high stereoselective aminobromination of β,β-dicyanostyrene derivatives with NBS as nitrogen/bromine source. Chin. J. Chem. 2012, 30, 830–836. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).