Abstract
3H-1,2-Dithiole-3-thiones are important compounds with many types of significant pharmacological activity. Although many derivatives of this class have been described in the literature, their thioethers have not previously been obtained. In this communication, it is shown that the reaction of 4,5-dichloro-3H-1,2-dithiole-3-thione with potassium isocyanate unexpectedly gave 5,5′-thiobis(4-chloro-3H-1,2-dithiole-3-thione). The structure of the synthesized compound was established by elemental analysis, high-resolution mass spectrometry, 13C NMR and IR spectroscopy, and mass spectrometry.
1. Introduction
3H-1,2-Dithiole-3-thiones show many types of significant biological activity [1] due to their ability to endogenously produce hydrogen sulfide (H2S), the third gaseous signaling molecule [2]. Among these heterocycles, readily available 4,5-dichloro-3H-1,2-dithiole-3-thione 1 [3] can be considered as an attractive starting material for many useful molecules due to its easy reactivity in 1,3-dipolar cycloadditions [4,5] and nucleophilic substitution of the 5-chlorine atom [6,7]. The introduction of a thiocyanate group into heterocyclic rings has led to various biological activities of these compounds [8]. We hypothesized that the combination of a 3H-1,2-dithiole-3-thione core with the thiocyanato group could give novel, biologically important compounds. Herein, we report the reaction of 4,5-dichloro-3H-1,2-dithiole-3-thione 1 with potassium isocyanate and the unexpected formation of 5,5’-thiobis(4-chloro-3H-1,2-dithiole-3-thione) 2 instead of 4-chloro-5-thiocyanato-3H-1,2-dithiole-3-thione 3.
2. Results and Discussion
In an attempt to obtain thiocyanate derivative 3, the reaction of 4,5-dichloro-3H-1,2-dithiole-3-thione 1 with potassium thiocyanate was studied. It was found that the treatment of compound 1 with potassium thiocyanate afforded in a mixture of aromatic hydrocarbon and water in the presence of tetrabutylammonium bromide (TBAB) as a phase transfer catalyst gave a new dimer 2: dark red solid C6Cl2S7 (Scheme 1). The reaction occurred when heated; the best result was obtained in toluene/water, where the starting material disappeared after 1 h (Table 1, Entry 6) and product 2 was isolated with a high yield (73%). With longer heating in benzene, along with the disappearance of the starting dithiolethione 1, the formation of decomposition products was observed.
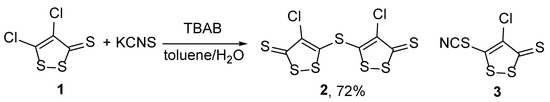
Scheme 1.
Reaction of 4,5-dichloro-3H-1,2-dithiole-3-thione 1 with potassium thiocyanate.

Table 1.
Reaction of 4,5-dichloro-3H-1,2-dithiole-3-thione 1 with KSCN.
We failed to detect the formation of thiocyanate derivative 3. It was not possible to obtain compound 3 at room temperature, since the reaction does not proceed under these conditions. Curiously, dimer 2 was not formed in the reaction of dithiolethione 1 with sodium sulfide when refluxing in a benzene/water mixture with TBAB for 1 h. Only the complete decomposition of starting material 1 was observed, and we failed to isolate individual products from the reaction mixture.
There are no data in the literature on the formation of heterocyclic sulfides from the corresponding thiocyanates. The only close analogy of the reaction presented in this paper is the formation of aromatic sulfides by the reductive coupling of aryl thiocyanates with aryl halides in the presence of SmI2 and a Pd catalyst [9].
The structure of 5,5′-thiobis(4-chloro-3H-1,2-dithiole-3-thione) 2 was fully confirmed by elemental analysis, high-resolution mass spectrometry, 13C NMR and IR spectroscopy, and mass spectrometry.
In conclusion, it is shown that the reaction of 4,5-dichloro-3H-1,2-dithiole-3-thione 1 with potassium thiocyanate leads to 5,5′-thiobis(4-chloro-3H-1,2-dithiole-3-thione) 2, which appears to be a dimerization product of 4-chloro-5-thiocyanato-3H-1,2-dithiol-3-thione 3. This compound may have useful pharmacological properties.
3. Materials and Methods
4,5-Dichloro-1,2-dithiole-3-thione 1 was prepared according to the published method [3]. The solvents and reagents were purchased from commercial sources and used as received. Elemental analysis was performed on a 2400 Elemental Analyzer (Perkin ElmerInc., Waltham, MA, USA). The melting point was determined on a Kofler hot-stage apparatus and was uncorrected. 13C NMR spectrum was taken with a Bruker AM-300 machine (Bruker AXS Handheld Inc., Kennewick, WA, USA) (at a frequency of 75 MHz) with TMS as the standard. The MS spectrum (EI, 70 eV) was obtained with a Finnigan MAT INCOS 50 instrument (Hazlet, NJ, USA). The IR spectrum was measured with a Bruker “Alpha-T” (Billerica, MA, USA) instrument in a KBr pellet. The high-resolution MS spectrum was measured on a Bruker micrOTOF II instrument (Bruker Daltonik Gmbh, Bremen, Germany) using electrospray ionization (ESI).
4,5-Dichloro-1,2-dithiole-3-thione 1. Red crystals, mp. 76 °C (lit. [3] mp. 76 °C). IR spectrum (KBr), ν, cm−1: 1704, 1660, 1532, 1272, 1080, 968, 848, 784. 13C-NMR (CDCl3, ppm): 136.0, 155.3, 202.9 (C=S). MS (EI, 70 Ev), m/z (I, %): 202 (M+, 23), 167 (M-Cl, 12), 135 (C3ClS2, 14), 103 (M-ClS2, 21). Anal. calcd for C3Cl2S3: C, 17.73; Cl, 34.98; S, 47.29%; found: C, 17.85; Cl, 34.90; S, 47.51%.
Synthesis of 5,5’-thiobis(4-chloro-3H-1,2-dithiole-3-thione) 2 (Supplementary Materials).
A solution of KCNS (97 mg, 1 mmol) in water (1 mL) and TBAB at the tip of a spatula were added to a solution of 4,5-dichloro-1,2-dithiol-3-thione 1 (203 mg, 1 mmol) in toluene (10 mL) and the reaction mixture was refluxed for 1 h (TLC). CH2Cl2 (50 mL) was added to the reaction mixture and the organic layer was separated, washed with water (5 mL), and dried over MgSO4. The residue was purified by column chromatography on silica gel (Silica gel Merck 60, eluent CH2Cl2). Yield 134 mg (73%), dark red crystals, mp. 149–150 °C. IR spectrum (KBr), ν, cm−1: 1632, 1439, 1265, 1235 (C=S), 1050 (C-Cl), 841. 13C-NMR (DMSO-d6, ppm): 137.7 (C-Cl), 157.6 (C-S), 205.4 (C=S). MS (EI, 70 Ev), m/z (I, %): 370 (M + 4, 22), 368 (M + 2, 62), 366 (M+, 71), 333 (5), 331 (8), 169 (5), 167 (15), 137 (43), 135 (100), 100 (90), 88 (34), 76 (26), 64 (26), 32 (13). HRMS (ESI-TOF): calcd for C6Cl2S7 [M + 1]− 366.7495; found m/z 366.7488. Anal. calcd for C6Cl2S7: C, 19.62; Cl, 19.30; found: C, 19.76; Cl, 19.11%.
Supplementary Materials
The following are available online: copies of 13C NMR, IR, HRMS and mass-spectra for the compound 2.
Author Contributions
Conceptualization, V.A.O.; methodology, O.A.R.; software, V.A.O.; validation, O.A.R.; formal analysis, investigation, V.A.O.; resources, O.A.R.; data curation, O.A.R.; writing—original draft preparation, V.A.O.; writing—review and editing, V.A.O.; visualization, O.A.R.; supervision, O.A.R.; project administration, O.A.R.; funding acquisition, O.A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 1 and 2 are available from the authors.
References
- Rakitin, O.A. Synthesis and reactivity of 3H-1,2-dithiole-3-thiones. Molecules 2021, 26, 3595. [Google Scholar] [CrossRef] [PubMed]
- Gadalla, M.M.; Snyder, S.H. Hydrogen sulfide as a gasotransmitter. J. Neurochem. 2010, 113, 14–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wentrup, G.-J.; Koepke, M.; Boberg, F. Über 1,2-Dithiacyclopentene; XXIX. 3-Thioxo-3H-1,2-Dithiole aus 3-Chloro-1,2-dithiolium-chloriden. Synthesis 1975, 525–526. [Google Scholar] [CrossRef]
- Ogurtsov, V.A.; Rakitin, O.A.; Rees, C.W.; Smolentsev, A.A.; Belyakov, P.A.; Golovanov, D.G.; Lyssenko, K.A. Synthesis of thienopyrantiones by a new molecular rearrangement. Org. Lett. 2005, 7, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsov, V.A.; Rakitin, O.A.; Rees, C.W.; Smolentsev, A.A. 4,5-Dichloro-1,2-dithiole-3-thione in the synthesis of benzimidazole, benzoxazole and benzothiazole derivatives of 1,3-dithioles. Mendeleev Commun. 2003, 13, 50–51. [Google Scholar] [CrossRef]
- Ogurtsov, V.A.; Rakitin, O.A.; Rees, C.W.; Smolentsev, A.A.; Lyssenko, K.A. New routes to 1,2-dithiole-3-thiones and 3-imines. Mendeleev Commun. 2005, 15, 20–21. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Kuzmich, A.S.; Sabutskii, Y.E.; Guzii, A.G.; Popov, R.S.; Ogurtsov, V.A.; Rakitin, O.A.; Polonik, S.G. Synthesis and studies of acetylthioglycoside conjugates of 4-chloro-1,2-dithiole-3-thione as potential antitumor agents. Russ. Chem. Bull. 2021, 70, 573–579. [Google Scholar] [CrossRef]
- Fortes, M.P.; da Silva, P.B.N.; da Silva, T.G.; Kaufman, T.S.; Militao, G.C.G.; Silveira, C.C. Synthesis and preliminary evaluation of 3-thiocyanato-1H-indoles as potential anticancer agents. Eur. J. Med. Chem. 2016, 118, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Still, I.W.J.; Dean, T.F. Reduction of aryl thiocyanates with SmI2 and Pd-catalised coupling with aryl halides as a route to mixed aryl sulfides. J. Org. Chem. 1996, 61, 7677–7680. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).