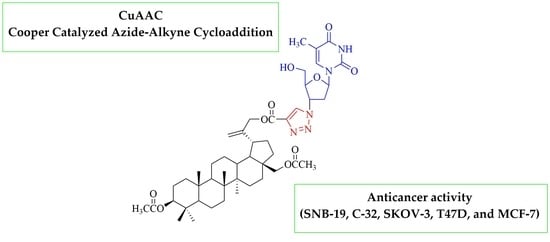
3′-[4-({[3β,28-Bis(acetyloxy)lup-20(29)-en-30-yl]oxy}carbonyl)-1H-1,2,3-triazol-1-yl]-3′-deoxythymidine
Abstract
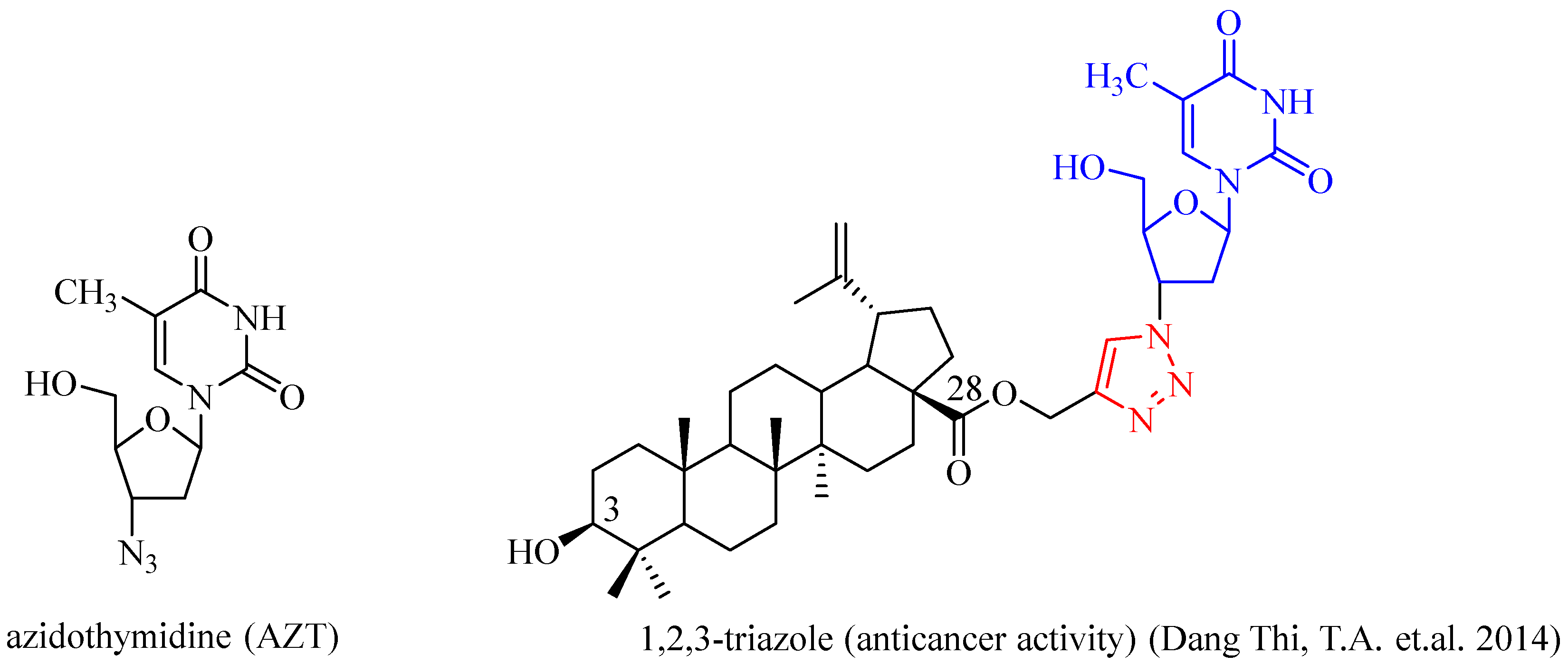
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Experimental Procedures
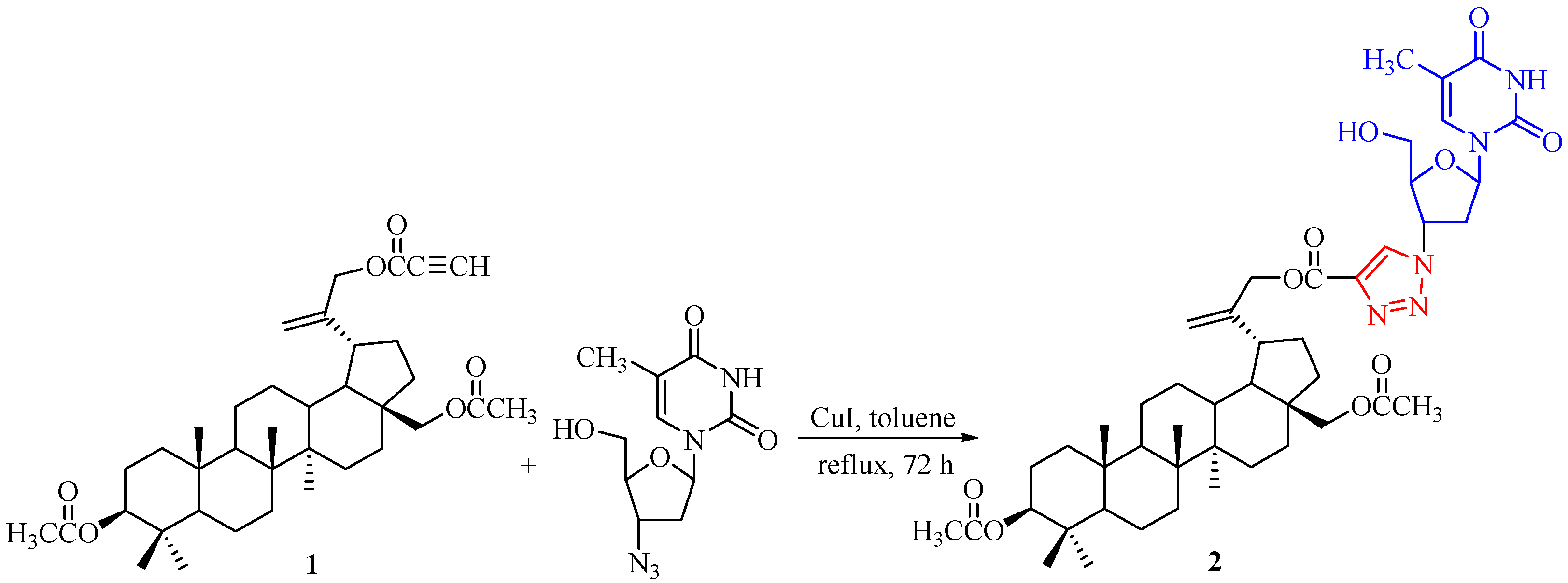
3.2. Synthesis of 3′-[4-({[3β,28-Bis(acetyloxy)lup-20(29)-en-30-yl]oxy}carbonyl)-1H-1,2,3-triazol-1-yl]-3′-deoxythymidine 2
Preparation of Compound 2:
3.3. Experimental Procedures
3.3.1. Cell Culture
3.3.2. WST-1 Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuczynska, K.; Bończak, B.; Rárová, L.; Kvasnicová, M.; Strnad, M.; Pakulski, Z.; Cmoch, P.; Fiałkowski, M. Synthesis and cytotoxic activity of 1,2,3-triazoles derived from 2,3-seco-dihydrobetulin via a click chemistry approach. J. Mol. Struct. 2022, 1250, 131751–131765. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, H.; Liu, J.; Liu, Y.; Wang, Y.; Xu, S.; Zhu, Z.; Xu, J. Synthesis, biological evaluation and mechanism studies of C-23 modified 23-hydroxybetulinic acid derivatives as anticancer agents. Eur. J. Med. Chem. 2019, 182, 111659. [Google Scholar] [CrossRef] [PubMed]
- Bębenek, E.; Chrobak, E.; Wietrzyk, J.; Kadela, M.; Chrobak, A.; Kusz, J.; Książek, M.; Jastrzębska, M.; Boryczka, S. Synthesis, structure and cytotoxic activity of acetylenic derivatives of betulonic and betulinic acids. J. Mol. Struc. 2016, 1106, 210–219. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Xu, R.; Wang, S.; Zhang, Y.; Zhang, L.; Zhou, D.; Xiao, S. Synthesis, structure activity relationship and in vitro anti-influenza virus activity of novel polyphenol-pentacyclic triterpene conjugates. Eur. J. Med. Chem. 2019, 163, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Du, X.N.; Li, Y.G.; Zhang, G.N.; Wang, J.X.; Wang, Y.C. Design, synthesis and biological evaluation of novel HIV-1 protease inhibitors with pentacyclic triterpenoids as P2-ligands. Bioorg. Med. Chem. Lett. 2019, 29, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Tu, B.; Liang, J.; Guo, S.; Cao, N.; Luo, Z.; Li, J.; Zheng, W.; Tang, X.; Li, D.; et al. Synthesis and biological evaluation of pentacyclic triterpenoid derivatives as potential novel antibacterial agents. Bioorg. Chem. 2021, 109, 104692–104705. [Google Scholar] [CrossRef] [PubMed]
- Catteau, L.; Zhu, L.; van Bambeke, F.; Quetin-Leclercq, J. Natural and hemi-synthetic pentacyclic triterpenes as antimicrobials and resistance modifying agents against Staphylococcus aureus: A review. Phytochem. Rev. 2018, 17, 1129–1163. [Google Scholar] [CrossRef]
- Fukumitsu, S.; Villareal, M.O.; Fujitsuka, T.; Aida, K.; Isoda, H. Anti-inflammatory and anti-arthritic effects of pentacyclic triterpenoids maslinic acid through NF-κB inactivation. Mol. Nutr. Food Res. 2016, 60, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Vo, N.N.Q.; Nomura, Y.; Muranaka, T.; Fukushima, E.O. Structure-activity relationships of pentacyclic triterpenoids as inhibitors of cyclooxygenase and lipoxygenase enzymes. J. Nat. Prod. 2019, 82, 3311–3320. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Han, N.; Wang, Y.W.; Zhai, J.X.; Liu, Z.H.; Liu, S.K.; Yin, J. Pentacyclic triterpenoid saponins, poterinasides A—J, from silverweed cinquefoil roots promising hepatoprotective and anti-inflammatory agents. J. Org. Chem. 2021, 86, 11220–11236. [Google Scholar] [CrossRef] [PubMed]
- Nejma, A.B.; Znati, M.; Daich, A.; Othman, M.; Lawson, A.M.; Jannet, H.B. Design and semisynthesis of new herbicide as 1,2,3-triazole derivatives of the natural maslinic acid. Steroids 2018, 138, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Li, M.; Yu, X.; Jin, H.; Zhang, Y.; Zhang, L.; Zhou, D.; Xiao, S. Synthesis and structure-activity relationship studies of water-soluble β-cyclodextrin-glycyrrhetinic acid conjugates as potential anti-influenza virus agents. Eur. J. Med. Chem. 2019, 166, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Dang Thi, T.A.; Kim Tuyet, N.T.; Pham The, C.; Thanh Nguyen, H.; Ba Thi, C.; Doan Duy, T.; D’hooghe, M.; van Nguyen, T. Synthesis and cytotoxic evaluation of novel ester-triazole-linked triterpenoid-AZT conjugates. Bioorg. Med. Chem. Lett. 2014, 24, 5190–5194. [Google Scholar] [CrossRef] [PubMed]
- Chrobak, E.; Bębenek, E.; Marciniec, K.; Kadela-Tomanek, M.; Siudak, S.; Latocha, M.; Boryczka, S. New 30-substituted derivatives of pentacyclic triterpenes: Preparation, biological activity, and molecular docking study. J. Mol. Struct. 2021, 1226, 129394–129404. [Google Scholar] [CrossRef]
- Bębenek, E.; Jastrzębska, M.; Kadela-Tomanek, M.; Chrobak, E.; Orzechowska, B.; Zwolińska, K.; Latocha, M.; Mertas, A.; Czuba, Z.; Boryczka, S. Novel triazole hybrids of betulin: Synthesis and biological activity profile. Molecules 2017, 22, 1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bębenek, E.; Kadela-Tomanek, M.; Chrobak, E.; Latocha, M.; Boryczka, S. Novel triazoles of 3-acetylbetulin and betulone as anticancer agents. Med. Chem. Res. 2018, 27, 2051–2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Compound | Human Cancer Cell Lines [IC50 ± SD] | ||||
|---|---|---|---|---|---|
| SNB-19 | C-32 | SKOV-3 | MCF-7 | T47D | |
| 2 | 19.42 ± 1.74 | 10.15 ± 1.03 | 11.97 ± 1.37 | 4.37 ± 0.52 | 32.62 ± 2.48 |
| Cisplatin | 13.10 ± 4.66 | 14.39 ± 2.33 | 36.77 ± 6.66 | 17.53 ± 2.66 | 31.50 ± 9.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bębenek, E.; Kadela-Tomanek, M.; Chrobak, E.; Latocha, M. 3′-[4-({[3β,28-Bis(acetyloxy)lup-20(29)-en-30-yl]oxy}carbonyl)-1H-1,2,3-triazol-1-yl]-3′-deoxythymidine. Molbank 2022, 2022, M1370. https://doi.org/10.3390/M1370
Bębenek E, Kadela-Tomanek M, Chrobak E, Latocha M. 3′-[4-({[3β,28-Bis(acetyloxy)lup-20(29)-en-30-yl]oxy}carbonyl)-1H-1,2,3-triazol-1-yl]-3′-deoxythymidine. Molbank. 2022; 2022(2):M1370. https://doi.org/10.3390/M1370
Chicago/Turabian StyleBębenek, Ewa, Monika Kadela-Tomanek, Elwira Chrobak, and Małgorzata Latocha. 2022. "3′-[4-({[3β,28-Bis(acetyloxy)lup-20(29)-en-30-yl]oxy}carbonyl)-1H-1,2,3-triazol-1-yl]-3′-deoxythymidine" Molbank 2022, no. 2: M1370. https://doi.org/10.3390/M1370
APA StyleBębenek, E., Kadela-Tomanek, M., Chrobak, E., & Latocha, M. (2022). 3′-[4-({[3β,28-Bis(acetyloxy)lup-20(29)-en-30-yl]oxy}carbonyl)-1H-1,2,3-triazol-1-yl]-3′-deoxythymidine. Molbank, 2022(2), M1370. https://doi.org/10.3390/M1370