Abstract
The X-ray structure of the title compound, obtained as a byproduct in a natural product synthesis, has been determined and shows an unusual pattern featuring chains of molecules with both intra- and intermolecular hydrogen bonding of the OH groups.
1. Introduction
Some time ago we described the isolation, structure determination and synthesis of an unusual trichlorodihydroxybibenzyl derived from a terrestrial plant native to Trinidad [1]. In the course of the synthesis, the corresponding symmetrical dichlorodihydroxybibenzyl 1 (Figure 1) was obtained as a byproduct and subjected to full spectroscopic characterisation. It was not new, however, since it had already been described much earlier in a 1957 publication by Pfleger and Waldmann [2] and also used in the synthesis of potential herbicides and algicides described in a 1978 patent [3]. Since the arrangement of hydroxyl groups present seemed to offer interesting opportunities for hydrogen bonding, we were interested to investigate this by means of an X-ray structure determination and we describe here the results.
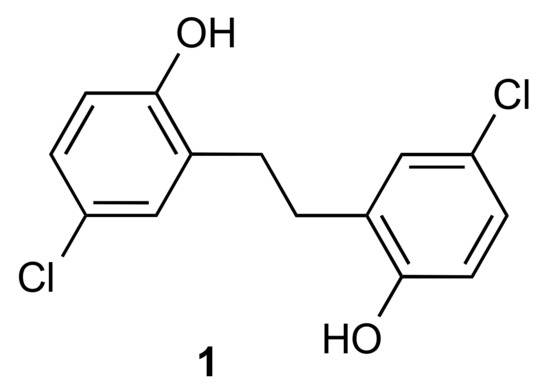
Figure 1.
Structure of the title compound 1.
2. Results
The compound was prepared as previously described [1] and was obtained directly by evaporation of a solution in ethanol as colourless prisms suitable for X-ray diffraction. The unit cell contained two closely similar independent molecules (the RMS between the two independent molecules, excluding H atoms is 0.11(1)), each involved in both intramolecular and intermolecular hydrogen bonding (Figure 2). The hydrogen bonding parameters are shown in Table 1.
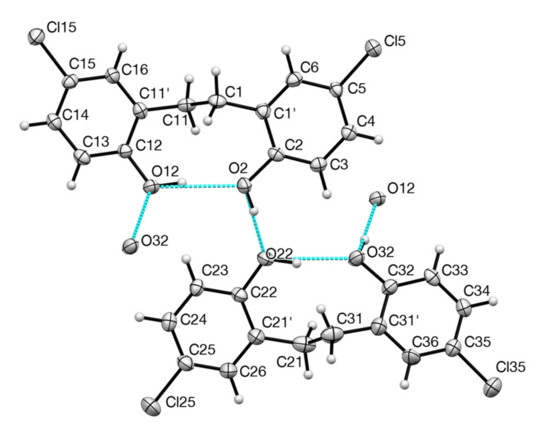
Figure 2.
Structure of the two independent molecules showing numbering and the basic hydrogen bonding pattern.

Table 1.
Hydrogen bonding parameters for 1 (Å, °).
A view of the unit cell along the a axis shows that the molecules form a series of hydrogen-bonded chains oriented in one of two orthogonal directions (Figure 3), and a view along the direction of these chains (Figure 4) clearly shows how the structure is built up by a combination of intra- and intermolecular hydrogen bonding. In terms of the Etter–Bernstein [4] graph set description, these are, respectively, S(9) and C22(18) interactions.
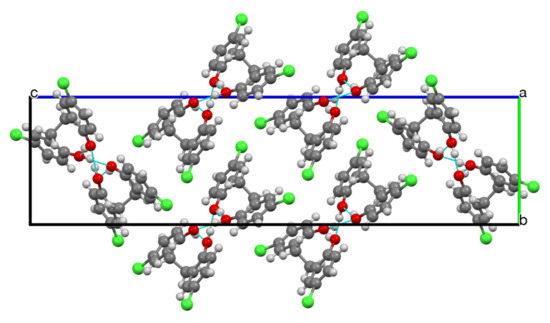
Figure 3.
Unit cell viewed along the a axis showing the arrangement of hydrogen-bonded chains.
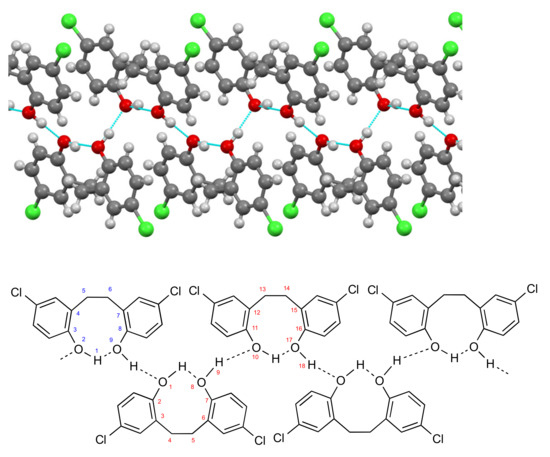
Figure 4.
Hydrogen bonding pattern for compound 1 viewed to avoid molecular overlap, together with schematic representation showing derivation of graph set descriptors.
A search of the Cambridge Structural Database (CSD) for 2,2-dihydroxybibenzyl-containing structures shows that the intramolecular S(9) interaction is very uncommon in these, but there is one structure (Refcode LESVEL) that displays this feature: the 2,3-bis(2-hydroxyphenyl)butane 2 [5]. However, rather than a chain as for 1, this actually forms dimers by an R22(8) interaction (Figure 5).
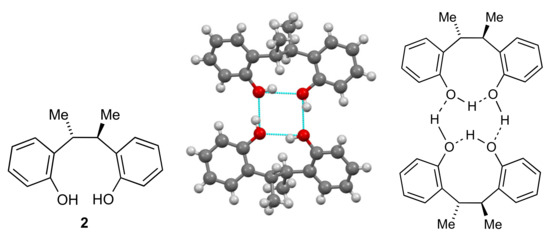
Figure 5.
Molecular and crystal structure of 2 [5].
In summary, the X-ray crystal structure of the title compound shows a series of parallel chains formed by intermolecular C22(18) hydrogen bonding interaction of pairs of slightly non-equivalent independent molecules each also featuring an intramolecular S(9) hydrogen bonded ring.
3. Experimental
Crystal data for C14H12Cl2O2, M = 283.15 g mol−1, colourless prism, crystal dimensions 0.03 × 0.03 × 0.03 mm, monoclinic, space group P21/c, a = 8.058(3), b = 9.064(3), c = 34.864(12) Å, β = 92.718(11)°, V = 2543.5(16) Å3, Z = 8, Dcalc = 1.479 g cm−3, T = 93 K, R1 = 0.0698, Rw2 = 0.17 for 3205 reflections with I > 2σ(I) and 341 variables. There are difference electron density peaks around the molecule containing Cl25 and Cl35 suggesting that there is a molecular disorder such as a C21–C31 crossover (pedal motion) but we were unable to resolve this disorder. The positions of hydrogen atoms bonding to carbon atoms are calculated geometrically and the coordinates and temperature factors are included in the refinement using the riding atom model. The positions of hydrogen atoms bound to oxygen atoms are found from the difference electron density and the coordinates and temperature factors are refined freely. Data were collected using graphite monochromated Mo Kα radiation λ = 0.71075 Å and have been deposited at the Cambridge Crystallographic Data Centre as CCDC 2128951. (Supplementary Materials). The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/getstructures. The structure was solved by direct methods and refined by full-matrix least-squares against F2 (SHELXL, Version 2018/3 [6]).
Supplementary Materials
The following supporting information can be downloaded online, cif and check-cif files for 1.
Author Contributions
A.L.G.G. prepared the compound; A.M.Z.S. collected the X-ray data and solved the structure; R.A.A. and R.S.R. designed the study; R.A.A. analysed the data and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The X-ray data are at CCDC as stated in the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aitken, R.A.; Bouquet, J.; Frank, J.; Gidlow, A.L.G.; Powder, Y.L.; Ramsewak, R.S.; Reynolds, W.F. Isolation, structure determination and synthesis of a trichlorodihydroxybibenzyl from a terrestrial plant. RSC Adv. 2013, 3, 7230–7232. [Google Scholar] [CrossRef]
- Pfleger, R.; Waldmann, K. Über den Einfluss der Äther-gruppen auf die Nitrierung im Bis(2-methoxy-5-chlor-phenyl)-sulfid und in Entsprechenden Diphenyl-alkanen. Chem. Ber. 1957, 90, 2395–2400. [Google Scholar] [CrossRef]
- Lacefield, W.B. Herbizide und Algizide. Ger. Pat. DE2738873 A1, 2 March 1978. [Google Scholar]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. Sect. B 1990, 46, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Fronczek, F.R. CCDC 626276: Experimental Crystal Structure Determination; The Cambridge Crystallographic Data Centre (CCDC): Cambridge, UK, 2007. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELXL. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).