1,3-Dimethyl-3′,5-diphenyl-1,5-dihydro-2H,5′H-spiro[furo[2,3-d]pyrimidine-6,4′-isoxazole]-2,4,5′(3H)-trione
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
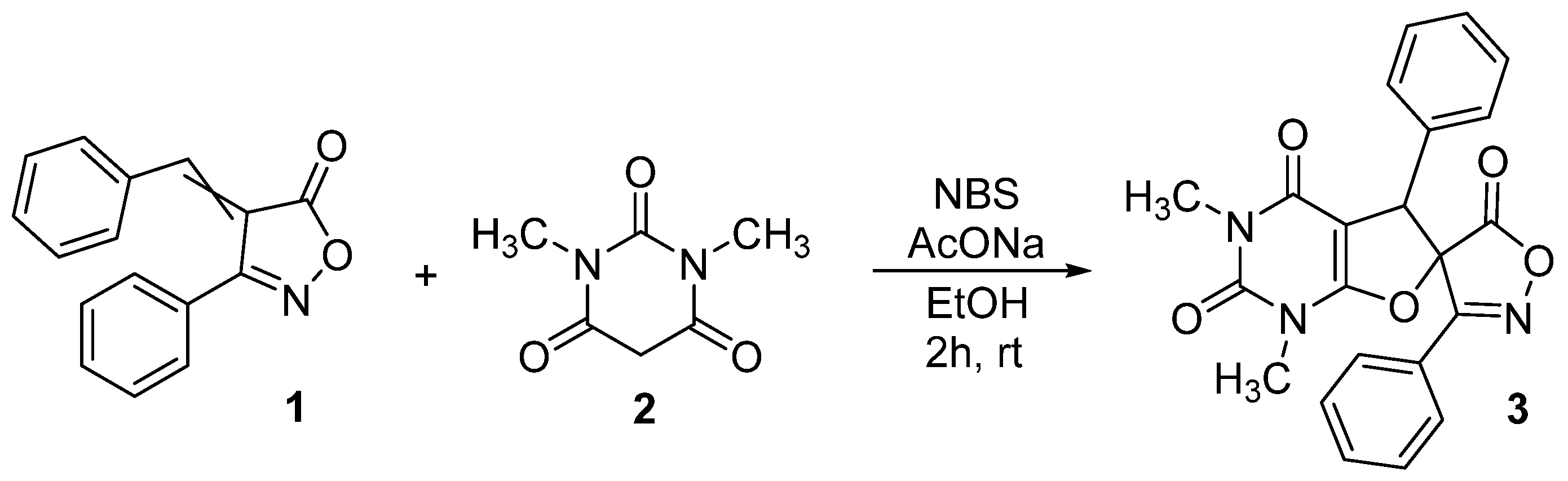
3.2. MHIRC Synthesis of 1,3-Dimethyl-3′,5-diphenyl-1,5-dihydro-2H,5′H-spiro[furo[2,3-d]pyrimidine-6,4′-isoxazole]-2,4,5′(3H)-trione 3
- 1,3-Dimethyl-3′,5-diphenyl-1,5-dihydro-2H,5′H-spiro[furo[2,3-d]pyrimidine-6,4′-isoxazole]-2,4,5′(3H)-trione (3). White solid; yield 86% (0.347 g); mp = 218–219 °C (decomp.) (from EtOH); and FTIR (KBr) cm−1: 3035 (C–H Ar), 1817 (C=O izox.), 1716 (C=O), 1683 (C=O), 1664 (C–C Ar), 1504 (C–C Ar), and 1050 (C–O–C). 1H NMR (300 MHz, DMSO-d6): δ 3.18 (s, 3H, N–CH3), 3.36 (s, 3H, N–CH3), 5.19 (s, 1H, CH), 7.12–7.20 (m, 2H, C(2)H and C(6)H Ph), 7.29–7.37 (m, 3H, C(3)H, C(4)H and C(5)H Ph), 7.64 (t, 3J = 7.2 Hz, 2H, C(3)H and C(5)H Ph izox.), 7.73 (t, 3J = 7.2 Hz, 1H, C(4)H Ph izox.), 7.91 (d, 3J = 7.2 Hz, 2H, C(2)H and C(6)H Ph izox.) ppm; 13C NMR (75 MHz, DMSO-d6): δ 27.8 (N(3)-Me), 29.7 (N(1)-Me), 53.8 (C(5)H), 85.4 (C spiro), 89.4 (C(4a)), 124.5 (C(1) Ph izox.), 127.1 (2C, C(2)H and C(6)H Ph), 128.4 (2C, C(3)H and C(5)H Ph izox.), 128.6 (2C, C(2)H and C(6)H Ph izox.), 128.7 (C(4)H Ph), 129.9 (2C, C(3)H and C(5)H Ph), 132.1 (C(1) Ph), 133.1 (C(4)H Ph izox.), 134.0 (C-Ph izox.), 158.2 (C(2)=O), 161.5 (C(1a)), 162.4 (C(4)=O), 169.6 (C=O izox.) ppm; MS (m/z, relative intensity %): 403 [M]+ (23), 358 [M-CO2-H]+ (9), 300 [M − C7H5N]+ (18), 284 [M − C7H5NO]+ (7), 243 [M − C9H5NO2 − H]+ (25), 142 [C9H4NO]+ (27), 77 [Ph]+ (100), 51 [C4H3]+ (76); Anal. calcd. for C22H17N3O5: C, 65.50; H, 4.25; N, 10.42%; found: C, 65.61; H, 4.29; N, 10.36%.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Little, R.D.; Dawson, J.R. MIRC (Michael Initiated Ring Closure) Reactions Formation of Three, Five, Six and Seven Membered Rings. Tetrahedron Lett. 1980, 21, 2609–2612. [Google Scholar] [CrossRef]
- Piras, L.; Moccia, M.; Cortigiani, M.; Adamo, M.F.A. Cyclopropanation of 5-(1-Bromo-2-phenyl-vinyl)-3-methyl-4-nitro-isoxazoles under Phase Transfer Catalysis (PTC) Conditions. Catalysts 2015, 5, 595–605. [Google Scholar] [CrossRef] [Green Version]
- Elinson, M.N.; Vereshchagin, A.N.; Stepanov, N.O.; Zaimovskaya, T.A.; Merkulova, V.M.; Nikishin, G.I. The first example of the cascade assembly of a spirocyclopropane structure: Direct transformation of benzylidenemalononitriles and N,N′-dialkylbarbituric acids into substituted 2-aryl-4,6,8-trioxo-5,7-diazaspiro[2.5]octane-1,1-dicarbonitriles. Tetrahedron Lett. 2010, 51, 428–431. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Rees, C.W.; Scriven, E.F.V. Comprehensive Heterocyclic Chemistry II; Pergamon Press: Oxford, UK, 1996; Available online: https://www.sciencedirect.com/referencework/9780080965185/comprehensive-heterocyclic-chemistry-ii#book-info (accessed on 29 December 2021).
- Ziarani, G.M.; Alealia, F.; Lashgari, N. Recent applications of barbituric acid in multicomponent reactions. RSC Adv. 2016, 6, 50895–50922. [Google Scholar] [CrossRef]
- Kliethermes, C.L.; Metten, P.; Belknap, J.K.; Buck, K.J.; Crabbe, J.C. Selection for pentobarbital withdrawal severity: Correlated differences in withdrawal from other sedative drugs. Brain Res. 2004, 1009, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Archana, A.; Srivastava, V.K.; Kumar, A. Synthesis of some newer derivatives of substituted quinazolinonyl-2-oxo/thiobarbituric acid as potent anticonvulsant agents. Bioorg. Med. Chem. 2004, 12, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Dhorajiya, B.D.; Dholakiya, B.Z.; Mohareb, R.M. Hybrid probes of aromatic amine and barbituric acid: Highly promising leads for anti-bacterial, anti-fungal and anti-cancer activities. Med. Chem. Res. 2014, 23, 3941–3952. [Google Scholar] [CrossRef]
- Sandberg, F. Anaesthetic Properties of Some New N-substituted and N,N′-disubstituted Derivatives of 5,5-Diallyl-Barbituric Acid. Acta Physiol. Scand. 1951, 24, 7–26. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kaur, M.; Verma, P. Design, synthesis and anticancer activities of hybrids of indole and barbituric acids—identification of highly promising leads. Bioorg. Med. Chem. Lett. 2009, 19, 3054–3058. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Mo, J.; Lin, H.; Chen, Y.; Sun, H. The recent progress of isoxazole in medicinal chemistry. Bioorg. Med. Chem. 2018, 26, 3065–3075. [Google Scholar] [CrossRef] [PubMed]
- Barmade, M.A.; Murumkar, P.R.; Sharma, M.K.; Yadav, M.R. Medicinal chemistry perspective of fused isoxazole derivatives. Curr. Top. Med. Chem. 2016, 16, 2863–2883. [Google Scholar] [CrossRef] [PubMed]
- Matloubi-Moghaddam, F.; Hojabri, L.; Taheri, S.; Pirani, P. A tandem aldol-diels-alder reaction accelerated in water: An approach to the catalyst-free one-pot synthesis of spiro thio-oxindoles. J. Iran. Chem. Soc. 2010, 7, 781–790. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Stepanov, N.O.; Belyakov, P.A.; Nikishin, G.I. Cascade assembly of N,N′-dialkylbarbituric acids and aldehydes: A simple and efficient one-pot approach to the substituted 1,5-dihydro-2H,2′H-spiro(furo[2,3-d]pyrimidine-6,5′-pyrimidine)-2,2′,4,4′,6′-(1′H,3H,3′H)-pentone framework. Tetrahedron Lett. 2010, 51, 6598–6601. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Dorofeeva, E.O.; Zaimovskaya, T.A.; Stepanov, N.O.; Gorbunov, S.V.; Belyakov, P.A.; Nikishin, G.I. Electrocatalytic and chemical assembling of N,N′-dialkylbarbituric acids and aldehydes: Efficient cascade approach to the spiro[furo[2,3-d]pyrimidine-6,5′-pyrimidine]-2,2′,4,4′,6′-(1′H,3H,3′H)-pentone framework. Tetrahedron 2012, 68, 1198–1206. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Dorofeeva, E.O.; Stepanov, N.O.; Zaimovskaya, T.A.; Nikishin, G.I. Electrocatalytic and chemical methods in MHIRC reactions: The first example of the multicomponent assembly of medicinally relevant spirocyclopropylbarbiturates from three different molecules. Tetrahedron 2013, 69, 1945–1952. [Google Scholar] [CrossRef]
- Elinson, M.N.; Dorofeeva, E.O.; Vereshchagin, A.N.; Nasybullin, R.F.; Egorov, M.P. Electrocatalytic stereoselective transformation of aldehydes and two molecules of pyrazolin-5-one into (R*,R*)-bis(spiro-2,4-dihydro-3H-pyrazol-3- one)cyclopropanes. Catal. Sci. Technol. 2015, 5, 2384–2387. [Google Scholar] [CrossRef]
- Ghorbani-Vaghei, R.; Maghbooli, Y. Synthesis of Activated Cyclopropanes by MHIRC Strategy: A Facile and Efficient Approach to Spirocyclopropanes Using N-Halosulfonamides. Synthesis 2016, 48, 3803–3811. [Google Scholar] [CrossRef]
- Grjol, B.; Jereb, M. Reactivity of substrates with multiple competitive reactive sites toward NBS under neat reaction conditions promoted by visible light. Chem. Pap. 2021, 75, 5235–5248. [Google Scholar] [CrossRef]
- Capreti, N.M.R.; Jurberg, I.D. Michael Addition of Soft Carbon Nucleophiles to Alkylidene Isoxazol-5-ones: A Divergent Entry to β-Branched Carbonyl Compounds. Org. Lett. 2015, 17, 2490–2493. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryzhkova, Y.E.; Kalashnikova, V.M.; Ryzhkov, F.V.; Elinson, M.N. 1,3-Dimethyl-3′,5-diphenyl-1,5-dihydro-2H,5′H-spiro[furo[2,3-d]pyrimidine-6,4′-isoxazole]-2,4,5′(3H)-trione. Molbank 2022, 2022, M1317. https://doi.org/10.3390/M1317
Ryzhkova YE, Kalashnikova VM, Ryzhkov FV, Elinson MN. 1,3-Dimethyl-3′,5-diphenyl-1,5-dihydro-2H,5′H-spiro[furo[2,3-d]pyrimidine-6,4′-isoxazole]-2,4,5′(3H)-trione. Molbank. 2022; 2022(1):M1317. https://doi.org/10.3390/M1317
Chicago/Turabian StyleRyzhkova, Yuliya E., Varvara M. Kalashnikova, Fedor V. Ryzhkov, and Michail N. Elinson. 2022. "1,3-Dimethyl-3′,5-diphenyl-1,5-dihydro-2H,5′H-spiro[furo[2,3-d]pyrimidine-6,4′-isoxazole]-2,4,5′(3H)-trione" Molbank 2022, no. 1: M1317. https://doi.org/10.3390/M1317
APA StyleRyzhkova, Y. E., Kalashnikova, V. M., Ryzhkov, F. V., & Elinson, M. N. (2022). 1,3-Dimethyl-3′,5-diphenyl-1,5-dihydro-2H,5′H-spiro[furo[2,3-d]pyrimidine-6,4′-isoxazole]-2,4,5′(3H)-trione. Molbank, 2022(1), M1317. https://doi.org/10.3390/M1317









