Abstract
2-(2-fluoro-[1,1′-biphenyl]-4-yl)-N-(1-phenylpropan-2-yl)propanamide was synthesized by a reaction between amphetamine and flurbiprofen in high yields. The newly obtained hybrid molecule was fully analyzed and characterized via 1H, 13C, UV, IR, HPLC, and mass spectral data.
1. Introduction
Discovered in 1910 by Barger and Dale, racemic α-methylphenethylamine (amphetamine) has transformed from a medicine that was freely available without the need of a prescription as a panacea for a broad range of disorders into a highly restricted and controlled medicine with therapeutic applications restricted to attention deficit hyperactivity disorder (ADHD) and narcolepsy (a sleep disorder) [1]. Amphetamine exerts its specific peripheral and central stimulant behavioral effects via the sustained elevation of extracellular concentrations of dopamine, serotonin, and norepinephrine [2]. Owing to a single chiral center, amphetamine exists in two optically active forms: d- (or R) and l- (or S) (d- for Dextro and l- for Levo-) (Figure 1), as more potent is d- or R-enantiomer.
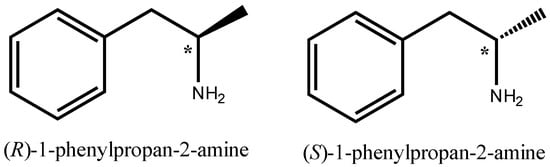
Figure 1.
Structural formulas of d- (or R) and l- (or S) amphetamine.
Amphetamine is a psychoactive substance that stimulates the central nervous system and creates a feeling of vigor, strength, and lightness, even in people in a state of severe physical exhaustion.
On the other hand, flurbiprofen (Figure 2) is a well-known nonsteroidal anti-inflammatory drug owing to anti-inflammatory, analgesic, and antipyretic activities, and has recently been used in many trials against viral infections [3] including SARS-CoV-2 [4,5].
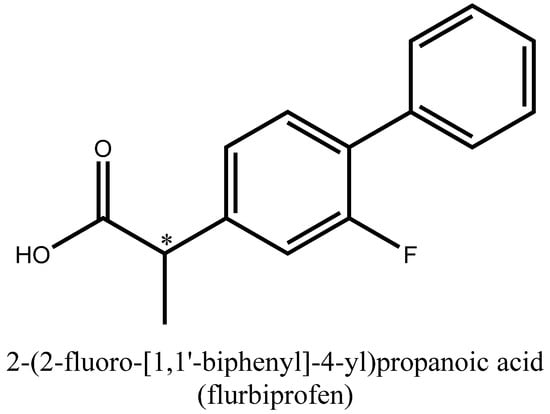
Figure 2.
Structural formula of flurbiprofen.
The building of amide functionality is of great importance in molecular sciences. The amide functional group is a critical bond that forms the backbone of peptides, proteins, and other biomolecules [6], considering its interaction with biological targets, especially with respect to N–H pKa and hydrogen bonding [7].
Amide synthesis is important for the pharmaceutical industry, in which the obtaining of amides is the most common chemical reaction employed [8].
Due to the importance of the creation of new amide hybrids in the pharmaceutical industry, a coupling reaction between racemic amphetamine and flurbiprofen was successfully achieved in order to obtain 2-(2-fluoro-[1,1′-biphenyl]-4-yl)-N-(1-phenylpropan-2-yl)propanamide.
2. Results
Herein, we report the successfully synthesized 2-(2-fluoro-[1,1′-biphenyl]-4-yl)-N-(1-phenylpropan-2-yl)propanamide 3, as shown in Scheme 1.
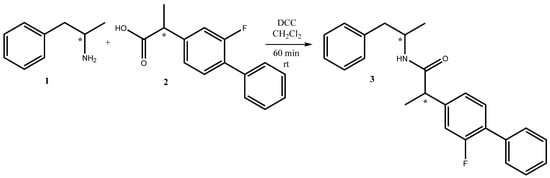
Scheme 1.
Synthesis of 2-(2-fluoro-[1,1′-biphenyl]-4-yl)-N-(1-phenylpropan-2-yl)propanamide 3.
An easy and handy synthetic procedure for amide synthesis is through N,N′-dicyclohexylcarbodiimide (DCC)-mediated coupling between amines and carboxylic acids (Scheme 1). DCC is a dehydrating agent commonly used for the preparation of esters, anhydrides, and amides. The mechanism of DCC is to activate the carboxylic acid (flurbiprofen 2) to the nucleophilic amphetamine 1. This reaction generally works in high yield.
The resultant compound is characterized by its melting point, 1H and 13C-NMR, UV, IR, HPLC, and HRMS spectra.
As each starting molecule provides a chiral carbon atom, the final structure possessed two chiral carbons. Therefore, we obtained a diastereoisomeric mixture of four stereoisomers. In two of them, the methyl groups will be in the cis- position, and in the other two, in the trans- position (Scheme 2).
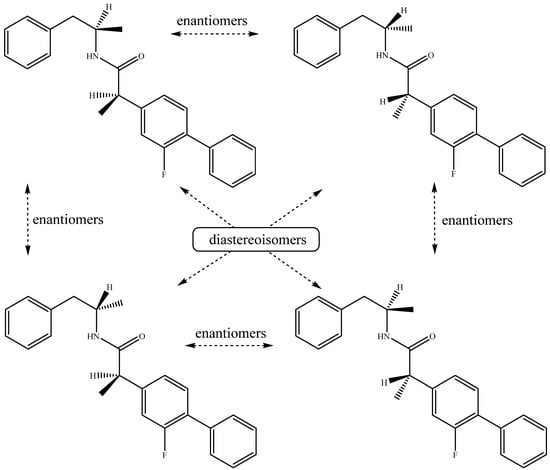
Scheme 2.
Stereoisomers of 2-(2-fluoro-[1,1′-biphenyl]-4-yl)-N-(1-phenylpropan-2-yl)propanamide 3.
We tried to separate the obtained diastereoisomers pairs but without success. Only one spot was observed on the thin layer chromatographic plate. Trying to separate the two pairs, we have performed HPLC analysis, and the results were the same. To separate the two stereoisomers, we used two methods of isocratic elution. In the formic acid method, the mobile phase composition was a mixture of 2% formic acid in water and 2% formic acid in acetonitrile used in ratio 95:5 v/v. The second method for elution of the mobile phase composition was a mixture of CH3CN:H2O:Et3N = 78:20:2 v/v. Formic acid and triethylamine were used in order to control the pH of the mobile phase. The application of the two methods practically did not lead to the separation of the two stereoisomers. We observed only one peak with a retention time of 3.95 min. Because of the fact that no conformational limitation is observed in the molecule, we were not able to separate the individual pairs. Examining the 1H-NMR spectra, we suppose we can see the two pairs of diastereoisomers. Starting from the left side, the two extra doublets are at 1.31 (d, J = 7.1 Hz) and 1.03 (d, J = 6.7 Hz). The absolute chemical shifts cannot be seen, just the number of peaks and their splitting. Those two have the same shape as the signals for the two methyl groups at 1.36 (d, J = 7.1 Hz, 3H) and 1.09 (d, J = 6.7 Hz, 3H), respectively (see Figure S1 in the Supplementary Materials file). Continuing along the spectrum, the signal for the CH2 group at 2.66 ppm is a doublet of doublet of doublets. A small peak of the middle of the main ddd indicates that half of the doublet of the weaker signal (ddd) overlaps with the stronger one. Here again, it can be seen that the shape and the number of the peaks correspond to the stronger ddd for the CH2 group (Figure S1a, page 3 in the Supplementary Materials file). The signal for the hydrogen next to the carbonyl carbon is a quartet. In Figure S1b, it is also observed that there is a smaller overlapped quartet, as the number of the resonant nuclei is 1.20. The next signal along the spectrum is 3.65–3.60 (m, 1H) for the hydrogen next to the nitrogen atom. The number of the resonant nuclei is 1.31, indicating that there is an overlapping signal for the H atom from one of the existing diastereoisomers. The UV spectra showed one λmax at 260 nm. The HRMS analysis unambiguously proves the authenticity of the structure.
The used method allows fast and easy synthesis of 2-(2-fluoro-[1,1′-biphenyl]-4-yl)-N-(1-phenylpropan-2-yl)propanamide 3 in high yield. Successfully obtained amide 3 is synthesized for the first time, and it is interesting because of its potential biological activity. The molecule contains on its structure proven pharmacophores, which are part of the structure of medicines used in medicinal practice.
3. Materials and Methods
All reagents and chemicals were purchased from commercial sources (Sigma-Aldrich S.A. and Riedel-de Haën, Sofia, Bulgaria) and used as received. Melting points were determined on a Boetius hot stage apparatus and are uncorrected. The NMR spectral data were recorded on a Bruker Avance II+600 spectrometer (BAS-IOCCP—Sofia, Bruker, Billerica, MA, USA). 1H-NMR and 13C-NMR spectra for compound 3 were taken in DMSO-d6 at 600 MHz and at 151 MHz, respectively. Chemical shifts are given in relative ppm and were referenced to tetramethylsilane (TMS) (δ = 0.00 ppm) as an internal standard; the coupling constants are indicated in Hz. The NMR spectra were recorded at room temperature (ac. 295 K). Mass analyses were carried out on a Q Exactive Plus mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). HPLC analysis consisted of quaternary mixer Smartline Manager 5000, pump Smartline 1000, and PDA 2800 detector (Knauer, Germany). Chromatographic conditions used: column Kromasil C18, 15 cm × 4.6 mm i.d., 5 µm particle size (Supelco, Bellefonte, PA, USA); mobile phase flow rate was set by 1.0 mL/min; sample volume was 20 µL. TLC was carried out on precoated 0.2 mm Fluka silica gel 60 plates (Merck KGaA, Darmstadt, Germany).
Synthesis of 2-(2-Fluoro-[1,1’-biphenyl]-4-yl)-N-(1-phenylpropan-2-yl)propanamide 3
N,N′-dicyclohexylcarbodiimide (1 mmol, 0.206 g) was added to a solution of flurbiprofen (1 mmol, 0.244 g) in CH2Cl2. The reaction mixture was stirred at room temperature for 10 min. After the addition of racemic amphetamine (1 mmol, 0.135 g), the reaction mixture was stirred for 50 min and the formation of white crystalline dicyclohexylurea was observed and then separated by filtration over a sintered glass filter. The filtrate was washed with a diluted hydrochloric acid, a saturated solution of Na2CO3, and brine. The combined organic layers were dried over anhydrous Na2SO4, and the solvent was removed under reduced pressure. Residual traces of dicyclohexylurea were removed after dissolving the sample in cold ethyl acetate. After 30 min, the crystallized dicyclohexylurea was removed by simple filtration. The compound was purified by short column chromatography.
2-(2-fluoro-[1,1’-biphenyl]-4-yl)-N-(1-phenylpropan-2-yl)propanamide3: white solid (m.p. 107–109 °C), yield 94% (0.338 g), 1H-NMR (600 MHz, DMSO) δ 7.59 (d, J = 7.9 Hz, 1H), 7.55–7.52 (m, 2H), 7.49–7.37 (m, 5H), 7.20 (t, J = 6.0 Hz, 1H), 7.14–7.04 (m, 5H), 4.02 (dt, J = 14.1, 6.9 Hz, 1H), 3.65–3.60 (m, 1H), 2.66 (ddd, J = 19.7, 13.5, 6.8 Hz, 2H), 1.36 (d, J = 7.1 Hz, 3H), 1.09 (d, J = 6.7 Hz, 3H). 13C-NMR (151 MHz, DMSO) δ 172.23 (C=O), 159.35 ((d, 1JC–F = 245.4 Hz), 139.23 (C, Ar), 135.70 (C, Ar), 130.62 (C, Ar), 129.55 (C, Ar), 129.49 (C, Ar), 129.05 (d, 3JC–F = 2.5 Hz), 129.04 (C, Ar), 128.95 (C, Ar), 128.44 (C, Ar), 128.27 (C, Ar), 128.02 (C, Ar), 126.21 (C, Ar), 124.14 (C, Ar), 115.24 (d, 2JC–F = 23.0 Hz), 46.30 (CH), 45.25 (CH2), 42.13 (CH), 20.55 (CH3), 18.61 (CH3). UV λmax, MeOH: 260 (ε = 60,800) nm. HRMS Electrospray ionization (ESI) m/z calcd for C24H25FNO+ = 262.1920, found 262.1918 (mass error Δm = −0.55 ppm). IR(KBr) νmax, cm−1: 3320 v(N–H, >NH), 3063 v(C–H, Ph), 3030 v(C–H, Ph), 2962 v(C–H, –CH3), 2929 v(C–H, >CH2), 1643 v(C=O), 1627 v(C–C=C, Ph), 1581 v(C–C=C, Ph), 1542 v(C–C=C, Ph), 1484 v(C–C=C, Ph), δ(>CH2), 1372 δ(–CH3), 767 γ(–C–H, Ph), 746 γ(–C–H, Ph), 698 γ(–C–H, Ph).
Copies of all spectra, HPLC chromatogram, and ESI-HRMS (Figures S1–S6) are provided in the Supplementary Materials file.
Supplementary Materials
The following supporting information can be downloaded online. Figure S1: 1H-NMR spectrum of compound 3; Figure S1a: Part of 1H-NMR spectrum of compound 3; Figure S1b: Part of 1H-NMR spectrum of compound 3; Figure S2: 13C-NMR spectrum of compound 3; Figure S3: UV spectrum of compound 3; Figure S4: ESI-HRMS of compound 3; Figure S5: IR spectrum of compound 3; Figure S6: HPLC chromatogram of compound 3.
Author Contributions
Conceptualization, I.I. and S.M.; methodology, S.M.; software, S.M.; validation, I.I., S.M. and D.B.; formal analysis, S.M. and D.B.; investigation, S.M.; resources, S.M.; data curation, S.M.; writing—original draft preparation, S.M.; writing—review and editing, I.I.; visualization, S.M.; supervision, I.I.; project administration, S.M.; funding acquisition, I.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Fund of the Bulgarian Ministry of Education and Science, grant number KΠ 06 M29/1.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article and supporting Supplementary Materials.
Acknowledgments
Dimitar Bojilov is thankful to the National Program of Ministry of Education and Science “Young Scientists and Postdoctoral Students” (PMC 577/2018) for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Heal, D.J.; Smith, S.J.; Gosden, J.; Nutt, D.J. Amphetamine, past and present—A pharmacological and clinical perspective. J. Psychopharmacol. 2013, 27, 479–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayan, A.J.; Aitken, B.; Downey, L.A.; Hayley, A.C. The effect of amphetamines alone and in combination with alcohol on functional neurocognition: A systematic review. Neurosci. Biobehav. Rev. 2021, 131, 865–881. [Google Scholar] [CrossRef] [PubMed]
- Shephard, A.; Smith, G.; Aspley, S.; Schachtel, B.P. Randomised, double-blind, placebo-controlled studies of flurbiprofen 8.75 mg lozenges in patients with/without group A or C streptococcal throat infection, with an assessment of clinicians’ predictions of strep throat. Int. J. Clin. Pract. 2015, 69, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, C.; Terrier, J.; Gloor, Y.; Curtin, F.; Rollason, V.; Desmeules, J.A.; Daali, Y.; Reny, J.-L.; Samer, C.F. Impact of SARS-CoV-2 infection (COVID-19) on cytochromes P450 activity assessed by the Geneva cocktail. Clin. Pharmacol. Ther. 2021, 110, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Capuano, A.; Scavone, C.; Racagni, G.; Scaglione, F. NSAIDs in patients with viral infections, including COVID-19: Victims or perpetrators? Pharmacol. Res. 2020, 157, 104849. [Google Scholar] [CrossRef] [PubMed]
- Sewald, N.; Jakubke, H.-D. Peptides: Chemistry and Biology; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Bray, B.L. Large-Scale manufacture of peptide therapeutics by chemical synthesis. Nat. Rev. Drug Discov. 2003, 2, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Seavill, P.W.; Wilden, J.D. The preparation and application of amides using electrosynthesis. Green Chem. 2020, 22, 7737–7759. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).