Abstract
N,N-Diarylthiophen-2-amine units are of great interest for the synthesis of optoelectronic devices. In this communication, N,N-bis (4′-(hexyloxy)-[1,1′-biphenyl]-4-yl)thiophen-2-amine was obtained by means of a Buchwald–Hartwig cross-coupling reaction of bis(4′-(hexyloxy)-[1,1′-biphenyl]-4-yl)amine and 2-bromothiophene in the presence of tris(dibenzylideneacetone)dipalladium(0), tri-tert-butyl phosphine and sodium tert-butanolate. The structure of newly synthesized compounds was established by means of elemental analysis, high-resolution mass spectrometry, 1H, 13C NMR, IR and UV spectroscopy and mass-spectrometry.
1. Introduction
The triarylamine donor is one of the most investigated in recent years for the synthesis of components organic solar cells (OSCs), organic light emitting diodes (OLEDs), organic field effect transistors (OFETs) and others [1,2]. In some cases, replacing the triarylamine unit with an N,N-diarylthiophen-2-amine can lead to better electron-donating ability, a higher short-circuit current density, and broadening the absorption range [3]. Based on this fragment, a number of compounds with a hole-transporting ability for applications in optoelectronic devices [4] and for designing small molecule donors for future optoelectronics [5], highly fluorescent luminogens [6] and advanced photosensitizer-based immunogenic cell death (ICD) inducers [7] were obtained.
However, among the previously synthesized derivatives of N,N-diarylthiophen-2-amine, there were no derivatives where Ar = biphenyl. It is known that the use of a biphenyl group in triarylamino-containing donors can significantly increase the photovoltaic characteristics of dyes [8,9]. Previously, we synthesized bis(4′-(hexyloxy)-[1,1′-biphenyl]-4-yl)amine 1 [10], which can be used as a precursor for the preparation of N,N-bis(4′-(hexyloxy)-[1,1′-biphenyl]-4-yl)thiophen-2-amine 2. Herein, we report the synthesis of this compound by Buchwald–Hartwig cross-coupling of bis(4′-(hexyloxy)-[1,1′-biphenyl]-4-yl)amine 1.
2. Results and Discussion
We conducted a study of the Buchwald–Hartwig cross-coupling reaction of bis(4′-(hexyloxy)-[1,1′-biphenyl]-4-yl)amine 1 with 2-bromothiophene 3 with the most frequently used N,N-bis(aryl)thiophen-2-amine and 2-bromothiophene catalysts—tris(dibenzylideneacetone)dipalladium(0), tri-tert-butyl phosphine and sodium tert-butanolate (Scheme 1, Table 1) [11,12,13]. The search for optimal reaction conditions was carried out by varying the nature of solvents and the temperature of chemical reactions. It was shown that when the reaction was carried out in boiling benzene, the yield of the reaction product 2 did not exceed 10% (Table 1, Entry 1). Replacement of benzene with a higher boiling toluene led to the introduction of a thienyl fragment into the amine 1 molecule within 24 h to obtain product 2, with a yield of 30% (Table 1, Entry 2). When using xylene as a solvent and heating the reaction mixture to 120 °C, the yield of the target product 2 reached to 45% (Table 1, Entry 3). A further increase in the reaction temperature to 130 °C did not lead to an improvement in the yield of compound 2 (Table 1, Entry 4).
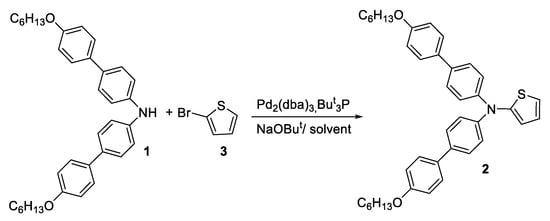
Scheme 1.
Synthesis of N,N-bis(4′-(hexyloxy)-[1,1′-biphenyl]-4-yl)thiophen-2-amine 2.

Table 1.
Reaction of bis(4′-(hexyloxy)-[1,1′-biphenyl]-4-yl)amine 1 with 2-bromothiophene 3.
The structure of N,N-bis(4′-(hexyloxy)-[1,1′-biphenyl]-4-yl)thiophen-2-amine 2 was confirmed by means of elemental analysis, high-resolution mass spectrometry, 1H, 13C NMR, IR and UV spectroscopy, and mass spectrometry.
3. Materials and Methods
Bis(4′-(hexyloxy)-[1,1′-biphenyl]-4-yl)amine 1 was prepared according to the published method [10]. The solvents and reagents were purchased from commercial sources and used as received. Elemental analysis was performed on a 2400 Elemental Analyzer (Perkin ElmerInc., Waltham MA, USA). The melting point was determined on a Kofler hot-stage apparatus and is uncorrected. 1H and 13C NMR spectra were taken with a Bruker AM-300 machine (Bruker AXS Handheld Inc., Kennewick WA, USA) (at frequencies of 300 and 75 MHz) in CDCl3 solution, with TMS as the standard. J values are given in Hz. The MS spectrum (EI, 70 eV) was obtained with a Finnigan MAT INCOS 50 instrument (Hazlet NJ, USA). The IR spectrum was measured with a Bruker “Alpha-T” instrument in KBr pellet. The high-resolution MS spectrum was measured on a Bruker micrOTOF II instrument (Bruker Daltonik Gmbh, Bremen, Germany) using electrospray ionization. The solution’s UV-visible absorption spectra were recorded using a OKB Spektr SF-2000 UV/Vis/NIR spectrophotometer controlled with SF-2000 software. The sample was measured in a 1 cm quartz cell at room temperature with 4.8 × 10−5 mol/mL concentration in CH2Cl2.
Synthesis of N,N-bis(4′-(hexyloxy)-[1,1′-biphenyl]-4-yl)thiophen-2-amine 2 (Supplementary Materials).
A mixture of bis(4′-(hexyloxy)-[1,1′-biphenyl]-4-yl)amine 1 (60 mg, 0.11 mmol), 2-bromothiophene 3 (28 mg, 0.17 mmol), NaOBut (16 mg, 0.17 mmol) and Pd2(dba)3 (2 mg, 2 mmol %) in xylene (5 mL) was degassed by argon and heated to 120 °C under argon for 20 h. On completion, the mixture was poured into water and extracted with CH2Cl2 (3 × 10 mL). The combined organic phases were washed with brine (2 × 5 mL), dried over MgSO4, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (silica gel Merck 60, eluent hexane–CH2Cl2, 2:1, v/v). Yield 30 mg (45%), yellow oil, Rf = 0.3 (hexane–CH2Cl2, 2:1, v/v). IR spectrum, ν, cm–1: 2953, 2928 and 2858 (all C–H), 1608, 1496, 1471, 1274, 1250, 1177, 820, 674, 516. 1H NMR (ppm): δ 7.53–7.47 (8H, m), 7.22 (d, J = 8.6, 4H), 7.05 (d, J = 4.8, 1H), 6.99–6.93 (5H, m), 6.81 (d, J = 3.5, 1H), 4.02 (t, J = 6.6, 4H), 1.83 (p, J = 6.7, 4H), 1.56–1.46 (m, 4), 1.42–1.37 (8H, m), 0.97–0.92 (6H, m). 13C NMR (ppm): δ 158.5, 151.3, 146.6, 135.4, 133.0, 127.7, 127.3, 126.0, 122.5, 121.5, 120.8, 114.8, 68.1, 31.6, 29.3, 25.7, 22.6, 14.0. HRMS (ESI-TOF), m/z: calcd for C40H46NO2S [M + H]+, 604.3244, found, 604.3228. MS (EI, 70eV), m/z (I, %): 606 ([M + 3]+, 3), 605 ([M + 2]+, 9), 604 ([M + 1]+, 60), 603 ([M]+, 100), 518 (12), 43 (5). UV-Vis spectra (in CH2Cl2), λmax: 267 nm (ε = 17,850 M−1 cm−1), 331 nm (ε = 22,402 M−1 cm−1). Anal. calcd. For C40H45NO2S (603.8568): C, 79.56; H, 7.51; N, 2.32. Found: C, 79.85; H, 7.72; N, 2.49%.
Supplementary Materials
The followings are available online: copies of1H, 13C NMR, IR, UV-Vis and mass-spectra for the compound 2.
Author Contributions
Conceptualization, O.A.R. and T.N.C.; methodology, O.A.R.; software, T.N.C.; validation, O.A.R.; formal analysis, T.N.C.; investigation, T.N.C.; resources, O.A.R.; data curation, O.A.R.; writing—Original draft preparation, O.A.R.; writing—Review and editing, O.A.R.; visualization, O.A.R.; supervision, O.A.R.; project administration, O.A.R.; funding acquisition, O.A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yahya, M.; Bouziani, A.; Ocak, C.; Seferoğlu, Z.; Sillanpää, M. Organic/metal-organic photosensitizers for dye-sensitized solar cells (DSSC): Recent developments, new trends, and future perceptions. Dye. Pigment. 2021, 192, 109227. [Google Scholar] [CrossRef]
- Knyazeva, E.A.; Rakitin, O.A. Influence of structural factors on the photovoltaic properties of dye-sensitized solar cells. Russ. Chem. Rev. 2016, 85, 1146–1183. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Li, X.; Mao, J.; Wu, W.; Ågren, H.; Hua, J. Photovoltaic properties of bis(octyloxy)benzo-[c][1,2,5]thiadiazole sensitizers based on an N,N-diphenylthiophen-2-amine donor. J. Mater. Chem. C 2014, 2, 4063–4072. [Google Scholar] [CrossRef]
- Capodilupo, A.-L.; Fabiano, E.; Franco, L.; Gambino, S.; Leoncini, M.; Accorsi, G.; Gigli, G. Control of Electron Transfer Processes in Multidimensional Arylamine-Based Mixed-Valence Compounds by Molecular Backbone Design. J. Phys. Chem. A 2021, 125, 7840–7851. [Google Scholar] [CrossRef] [PubMed]
- Choua, S.-H.; Chen, H.-C.; Wang, C.-K.; Chung, C.-L.; Hung, C.-M.; Hsu, J.-C.; Wong, K.-T. Synthesis and characterization of new asymmetric thieno [3,4-b]pyrazinebased D−π−A−A type small molecular donors with near-infrared absorption and their photovoltaic applications. Org. Electron. 2019, 68, 159–167. [Google Scholar] [CrossRef]
- Chen, C.-H.; Luo, Z.-H.; Huan, I.-H.; Chen, Y.-H.; Lim, T.-S. Rationalize the roles of electron donating-withdrawing groups in the impacts on solvatochromism, nonlinear optics, and electroluminescence devices. Dye. Pigment. 2020, 175, 10843. [Google Scholar] [CrossRef]
- Chen, C.; Ni, X.; Jia, S.; Liang, Y.; Wu, X.; Kong, D.; Ding, D. Massively Evoking Immunogenic Cell Death by Focused Mitochondrial Oxidative Stress using an AIE Luminogen with a Twisted Molecular Structure. Adv. Mater. 2019, 31, 1904914. [Google Scholar] [CrossRef] [PubMed]
- Wild, M.; Griebel, J.; Hajduk, A.; Friedrich, D.; Stark, A.; Abel, B.; Siefermann, K.R. Efficient synthesis of triarylaminebased dyes for p-type dyesensitized solar cells. Sci. Rep. 2016, 6, 26263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Zhou, H.; Zhang, S.; Gea, C.; Cheng, S. Influence of the auxiliary acceptor and π-bridge in triarylamine dyes on dye-sensitized solar cells. Photochem. Photobiol. Sci. 2019, 18, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Chmovzh, T.N.; Rakitin, O.A. tert-Butyl Bis(4′-(hexyloxy)-[1,1′-biphenyl]-4-yl)carbamate. Molbank 2021, 2021, M1247. [Google Scholar] [CrossRef]
- Watanabe, M.; Yamamoto, T.; Nishiyama, M. Synthesis of novel (bis)(diarylamino)thiophenes via palladium-catalysed reaction of (di)bromothiophenes with diarylamines. Chem. Commun. 2000, 2, 133–134. [Google Scholar] [CrossRef]
- Capodilupo, A.-L.; Vergaro, V.; Accorsi, G.; Fabiano, E.; Baldassarre, F.; Corrente, G.A.; Gigli, G.; Ciccarella, G. A series of diphenylamine-fluorenone derivatives as potential fluorescent probes for neuroblastoma cell staining. Tetrahedron 2016, 72, 2920–2928. [Google Scholar] [CrossRef]
- Shimogawa, H.; Murata, Y.; Wakamiya, A. NIR-Absorbing Dye Based on BF2-Bridged Azafulvene Dimer as a Strong Electron-Accepting Unit. Org. Lett. 2018, 20, 5135–5138. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).