Abstract
5-propyl-2-((trityloxy)methyl)thiophene-3-carbaldehyde was synthesized by using the concept of chemo- and regioselective Br/Li exchange reaction from 3-bromo-5-propyl-2-((trityloxy)methyl)thiophene. This is a five-step protocol starting from thiophene with an overall yield of 33%. These lithium/halogen exchange reactions were carried out at −78 °C to rt over the period of 1 to 18 h depending on the reactivity of electrophiles.
1. Introduction
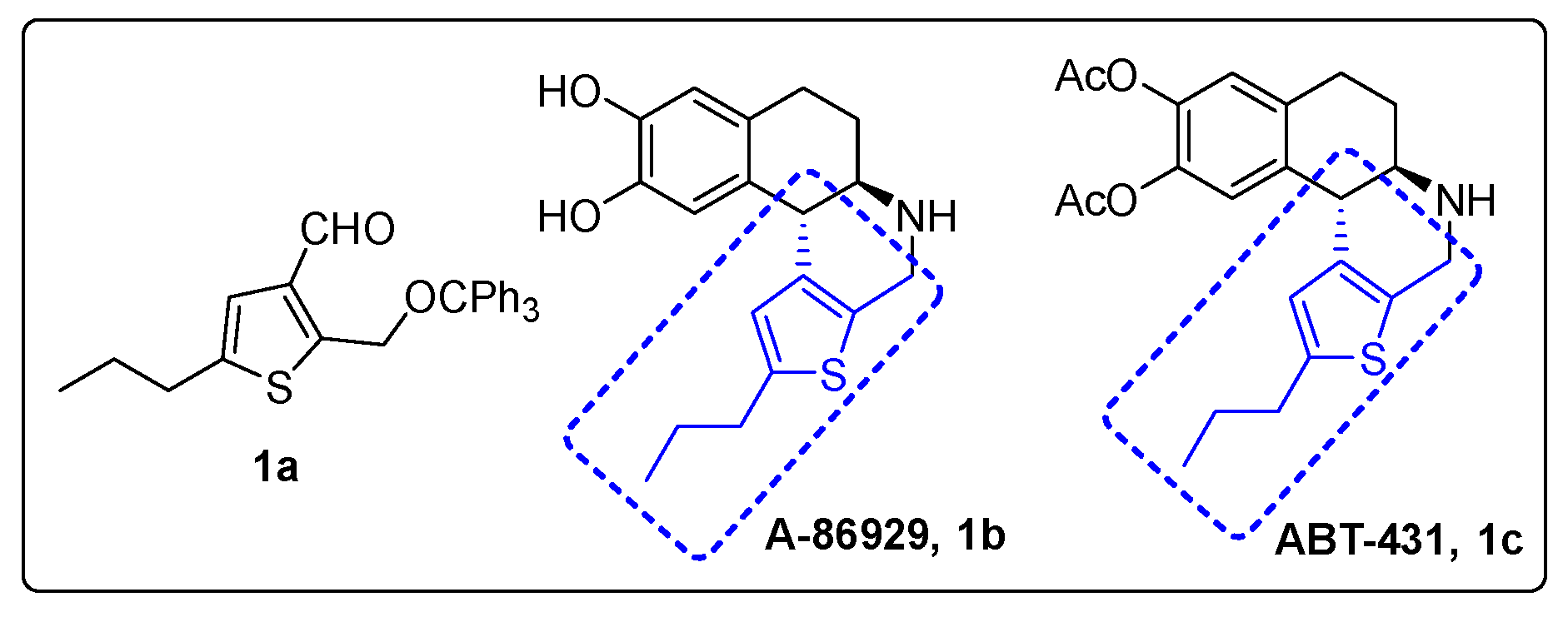
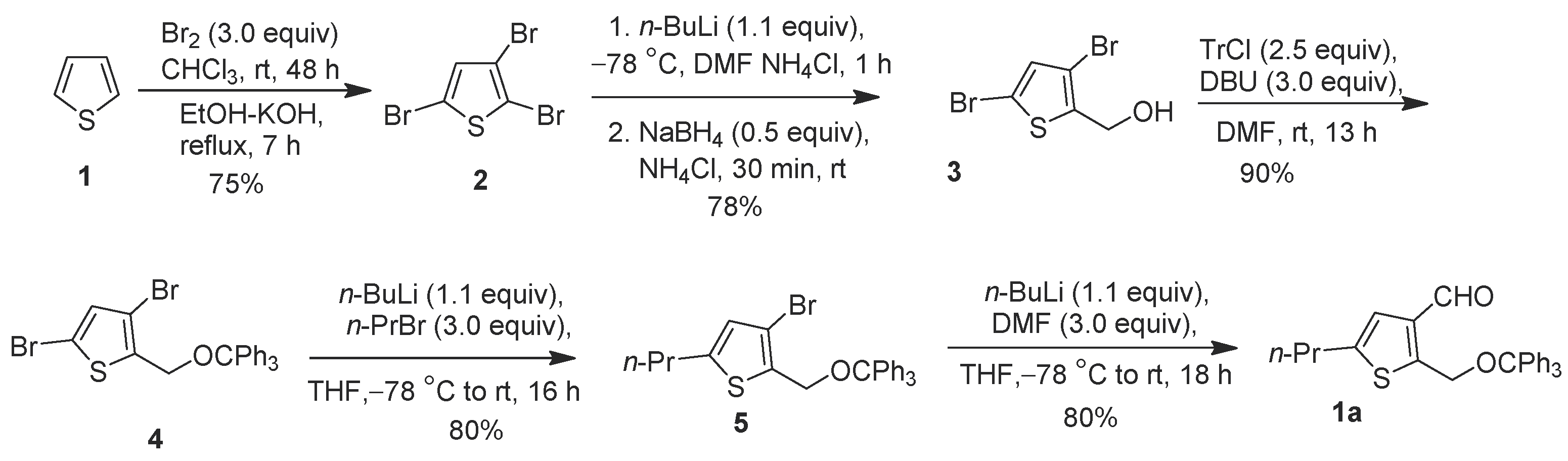
Alkylated thiophene-3-carbaldehyde is a widely used building block for organic synthesis of a myriad of materials with multiple applications. It has been used as the key starting material for organic field-effect transistors [1,2,3,4,5], glucagon antagonist [6], inhibitors of D-amino acid oxidase [7], dopamine 1 agonist ABT-431 (Figure 1) [8,9], and water-soluble fluorescent polymers [10]. Due to their strong luminescence properties, thiophene-based compounds have also been used for dye-sensitized organic solar cells and light emitting devices [11] as well as nonlinear optical chromophores [12]. Thiophene-derived small organic molecules have shown to possess many biological applications, such as antitumor [13], analgesic [14], and anti-inflammatory [15] treatments. One example compound of interest is dopamine d1 adonist ABT-431 (Figure 1), which consists of a trialkyl-substituted thiophene that could be readily synthesized with an accessibly derivatized thiophene. 5-Alkyl-2-((trityloxy)methyl)thiophene-3-carbaldehyde is a very versatile organic molecule by nature, with functionalizable handles that allow facile derivation. The aldehyde group in position 3 allows for immediate reactivity, while a methyl-alcohol can be exposed via deprotection of the trityl group. This system also benefits from its flexibility at position 5, being capable of most stable alkyl functionalizations. In the present study, we report a concise five-step synthesis protocol for a new thiophene derivative, 5-propyl-2-((trityloxy)methyl)thiophene-3-carbaldehyde 1a, starting from thiophene (Scheme 1).
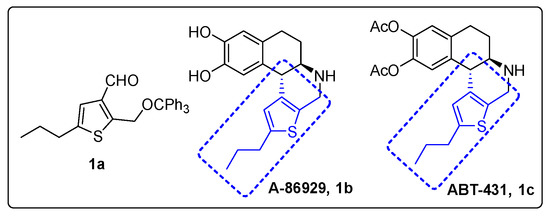
Figure 1.
Thiophene fragment in A-86929 and ABT-431.
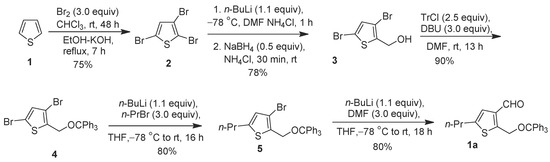
Scheme 1.
Synthesis of 5-propyl-2-((trityloxy)methyl)thiophene-3-carbaldehyde 1a.
2. Results and Discussion
5-Propyl-2-((trityloxy)methyl)thiophene-3-carbaldehyde (1a) was prepared from 2,3,5-tribromothiophene (2) by three consecutive chemo- and regioselective lithium/bromine exchange reactions, as shown in Scheme 1. 2,3,5-Tribromothiophene 2 was prepared by bromination of thiophene 1 by using an excess of Br2 (3 equiv.) in CHCl3 following the literature procedure [16] and gave a 75% yield following a 48 h reaction time and purification.
The lithium/bromine exchange reaction of 2 was performed using n-BuLi and, trapping with DMF, provided chemo- and regioselective 3,5-dibromo-2-thiophenecarboxaldehyde at −78 °C in THF. This crude 3,5-dibromo-2-thiophenecarboxaldehyde was reduced with NaBH4 to form 3,5-dibromothiophen-2-methanol (3) in an excellent yield of 78%. Once the thiophene derivative 3 was isolated, it was taken forward to the next step. Trityl protection was carried out by using trityl chloride and DBU in DMF at rt for 13 h to obtain 3,5-dibromo-2-((trityloxy)methyl)thiophene (4) with an excellent yield of 90%.
Next, n-BuLi mediated the propylation of 4, and using n-propyl bromide at −78 °C in THF afforded 3-bromo-5-propyl-2-trityloxymethylthiophene (1a) in an 80% yield [8]. One final lithium/bromine exchange reaction using n-BuLi at −78 °C was conducted and the subsequent quenching with DMF provided 1a in an 80% yield.
3. Materials and Methods
All reactions were conducted using oven-dried glassware under an atmosphere of Argon (Ar). Commercial grade reagents were used without further purification. Commercially available anhydrous solvents were used. Flash chromatography was carried out using silica gel (230–400 mesh). TLC was performed on aluminum-backed plates coated with silica gel 60 with F254 indicator. The 1H-NMR spectra were recorded with a 200 MHz NMR and the 13C-NMR spectrum was recorded with a 50 MHz NMR at the Department of Chemistry, Indian Institute of Technology, Kharagpur, India. 1H-NMR chemical shifts are expressed in parts per million (δ) relative to CDCl3 (δ = 7.26). 13C-NMR chemical shifts are expressed in parts per million (δ) relative to the CDCl3 resonance (δ = 77.0). Melting points (m.p.) of solid compounds are reported without correction. Elemental analysis (CHN) was recorded at Department of Chemistry, Indian Institute of Technology, Kharagpur, India. 1H and 13C-NMR spectra can be found in the supplementary materials section.
3.1. Synthesis of 2,3,5-Tribromothiophene 2
Thiophene 1 (134 mmol, 11.25 g) and 200 mL of chloroform were charged into a 500 mL, three-necked flask equipped with a stirrer, a dropping funnel, and an outlet for the hydrogen bromide evolution. Bromine (406 mmol, 64.80 g) was added dropwise to the stirred mixture over a period of 2 h in ice cold conditions. Then, the reaction mixture was warmed to room temperature. After the mixture rested overnight, it was heated at 50 °C for 24 h, washed with a 2N sodium hydroxide solution, refluxed for 7 h with a solution of 8.0 g of potassium hydroxide in 150 mL of 95% ethanol, and poured into water. The organic layer was separated, washed with water, dried over Na2SO4, and solvent evaporation gave the titled compound as a light-yellow solid (32 g, 75% yield). 1H-NMR (CDCl3, 200 MHz): 6.90 (s, 1H).
3.2. Synthesis of (3,5-Dibromothiophen-2-yl)methanol 3
2,3,5-Tribromothiophene 2 (93 mmol, 30 g) and 300 mL of dry THF were taken in 500 mL of a round bottom flask and cooled to −78 °C. A total of 66 mL of n-BuLi (1.1 equiv., 1.6 M in hexane) was added slowly to the reaction mixture over a period of 10 min and the mixture was stirred for 30 min at the same temperature. Then, 18.0 g of DMF was added slowly to the reaction mixture and stirred at −78 °C for 30 min. It was warmed to 0 °C and quenched with saturated NH4Cl solution. The reaction mixture was extracted with EtOAc, dried with Na2SO4, and concentrated to obtain a light-yellow liquid. A total of 100 mL of THF was added to it and the reaction mixture was placed at 0 °C. An amount of 8 mL of methanol was added and then 6 g of NaBH4 was added portion-wise. After being stirred for 20 min at room temperature, the reaction mixture was quenched with NH4Cl and extracted with EtOAc. The organic layer was washed with brine and dried over Na2SO4. Concentration and flash column chromatography (ethyl acetate/hexane) gave the titled compound as a light-yellow liquid (19.2 g, 78% yield). 1H-NMR (CDCl3, 200 MHz): 6.91 (s, 1H), 4.72 (s, 2H), 1.98 (bs, 2H).
3.3. Synthesis of 3,5-Dibromo-2-((trityloxy)methyl)thiophene 4
To a solution of 3,5-dibromothiophen-2-methanol 3 (54.0 mmol, 14.2 g) and DBU (189 mmol, 28.7 g) in DMF (54 mL) was added tritylchloride (45.0 g, 162.0 mmol) at rt. After stirring for 13 h, the reaction mixture was diluted with EtOAc. The organic layer was washed with 10% HCl, NaHCO3, and brine, and then dried over Na2SO4. Concentration and column purification with hexane gave the titled compound as a white solid (25.2 g, 90% yield). m.p.: 118 °C. 1H-NMR (CDCl3, 200 MHz): 7.54–7.47 (m, 6H), 7.37–7.21 (m, 9H), 6.78 (s, 1H), 4.31, (s, 2H).
3.4. Synthesis of 3-Bromo-5-propyl-2-((trityloxy)methyl)thiophene 5
3,5-Dibromo-2-trityloxymethylthiophene 4 (20.0 mmol, 10.3 g) and 80 mL of THF were taken in a 250 mL round bottom flask and placed at −78 °C. A total of 20 mL of n-BuLi (22.0 mmol, 1.6 M in hexane) was added dropwise over 10 min and stirred for 20 min. Freshly distilled propyl bromide (3 equiv.) was added over 5 min at that temperature and warmed to rt. The solution was stirred for 16 h at rt. The reaction was quenched with H2O and the mixture was extracted with EtOAc. The organic layer was washed with brine and dried over Na2SO4. Concentration, flash column purification from hexane, and recrystallization (toluene/ethanol) gave the titled compound as a colorless solid (7.5 g, 80% yield). m.p.: 92 °C. 1H-NMR (CDCl3, 200 MHz): 7.54–7.47 (m, 6H), 7.37–7.21 (m, 9H), 6.60 (s, 1H), 4.21, (s, 2H), 2.75 (t, J = 7.4 Hz, 2H), 1.80–1.60 (m, 2H), 0.99 (t, J = 7.2 Hz, 3H).
3.5. Synthesis of 5-Propyl-2-((trityloxy)methyl)thiophene-3-carbaldehyde 1a
3-Bromo-5-propyl-2-trityloxymethyl-thiophene 5 (14.70 mmol, 7.0 g) and 60 mL of THF were taken in a 100 mL round bottom flask and placed at −78 °C. An amount of 10.1 mL of n-BuLi (16.17 mmol, 1.6 M in hexane) was added dropwise over 10 min and stirred for 20 min. Neat DMF (3.3 g, 44.1 mmol) was added slowly over 5 min and the reaction was stirred at rt for 18 h. On completion, it was quenched with NH4Cl and extracted with ethyl acetate. Flash column purification (ethyl acetate/hexane) gave the titled compound as a colorless solid (5.0 g, 80% yield). m.p.: 100 °C. 1H-NMR (CDCl3, 200 MHz): 9.70 (s, 1H), 7.52–7.48 (m, 6H), 7.37–7.22 (m, 9H), 7.06 (s, 1H), 4.62 (s, 2H), 2.77 (t, J = 7.4 Hz, 2H), 1.83–1.61 (m, 2H), 0.98 (t, J = 7.2 Hz, 3H). 13C-NMR (CDCl3, 50 MHz): 184.5, 151.9, 144.4, 143.4, 136.1, 128.59, 128.0, 127.3, 123.8, 87.8, 60.68, 31.8, 24.5, 13.6. Anal. (CHN %) Calcd for C28H26O2S: C, 78.84; H, 6.14 Found C, 78.79; H, 6.19.
4. Conclusions
This protocol utilizes the chemo- and regioselective Br/Li exchange reaction to generate multi-functionalized thiophene, which can then be utilized for further derivation with numerous applications. Specifically, up to 5 g of 5-propyl-2-((trityloxy)methyl)thiophene-3-carbaldehyde was synthesized from thiophene in only 5 steps with an overall yield of 33%.
Supplementary Materials
The following are available online: Figure S1: 1H-NMR spectrum of compound 2; Figure S2: 1H-NMR spectrum of compound 3; Figure S3: 1H-NMR spectrum of compound 5; Figure S4: 1H-NMR spectrum of compound 1a; and Figure S5: 13C-NMR spectrum of compound 1a.
Funding
This research received funding from DST, New Delhi, India.
Data Availability Statement
The data presented in this study are available in this article and supporting Supplementary Materials.
Acknowledgments
I would like to thank my mentor, Saumen Hajra, for his continuous support throughout my career.
Conflicts of Interest
The author declares no conflict of interest.
References
- Hyodo, K.; Toyama, R.; Hiroki Mori, H.; Yasushi Nishihara, Y. Synthesis and physicochemical properties of piceno[4,3-b:9,10-b′]dithiophene derivatives and their application in organic field-effect transistors. ACS Omega 2017, 2, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Author Huang, P.-Y.; Kim, C.; Chen, M.-C. First tetrabutylanthradithiophene (TBADT) derivatives for solution-processed thin-film transistors. Synlett 2011, 15, 2151–2156. [Google Scholar]
- Nishinaga, S.; Mori, H.; Nishihara, Y. Synthesis and transistor application of bis[1]benzothieno[6,7-d:6′,7′-d′]benzo[1,2-b:4,5-b′]dithiophenes. J. Org. Chem. 2018, 83, 5506–5515. [Google Scholar] [CrossRef] [PubMed]
- Koumura, N.; Wang, Z.S.; Mori, S.; Miyashita, M.; Suzuki, E.; Hara, J.K. Alkyl-functionalized organic dyes for efficient molecular photovoltaics. J. Am. Chem. Soc. 2006, 128, 14256–14257. [Google Scholar] [CrossRef] [PubMed]
- Thayumanavan, S.; Mendez, J.; Marder, S.R. Synthesis of functionalized organic second-order nonlinear optical chromophores for electro-optic applications. J. Org. Chem. 1999, 64, 4289–4297. [Google Scholar] [CrossRef]
- Li, J.; Feng, Y.; Li, H.; Shu, S.; Dai, A.; Cai, X.; Wang, J.; Yang, D.; Ma, D.; Wang, M.-W.; et al. Discovery of thiophene-containing biaryl amide derivatives as novel glucagon receptor antagonists. Chem. Biol. Drug Des. 2018, 92, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Hin, H.; Maita, N.; Thomas, A.G.; Kurosawa, S.; Rojas, C.; Yorita, K.; Barbara, S.S.; Fukui, K.; Tsukamoto, T. Structural basis for potent inhibition of D-amino acid oxidase bythiophene carboxylic acids. Eur. J. Med. Chem. 2018, 159, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Yamada, K.; Tomioka, K. Construction of arene-fused-piperidine motifs by asymmetric addition of 2-trityloxymethylaryllithiums to nitroalkenes: The asymmetric synthesis of a dopamine D1 full agonist, A-86929. J. Am. Chem. Soc. 2004, 126, 1954–1955. [Google Scholar] [CrossRef] [PubMed]
- Bar, S.; Hajra, S. Catalytic enantioselective synthesis of A-86929, a dopamine D1 agonist. Chem. Commun. 2011, 47, 3981–3982. [Google Scholar]
- Altinok, E.; Frausto, F.; Thomas III, S.W. Water-soluble fluorescent polymers that respond to singlet oxygen. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2526–2535. [Google Scholar] [CrossRef]
- Garnier, F. Functionalized conducting polymer. Angew. Chem. Int. Ed. Engl. 1989, 28, 513–517. [Google Scholar] [CrossRef]
- Han, P.; Yang, Z.; Cao, H.; He, W.; Wang, D.; Zhang, J.; Xing, Y.; Gao, H. Nonlinear optical properties of the novel kind of organic donor-acceptor thiophene derivatives with click chemistry modification. Tetrahedron 2017, 73, 6210–6216. [Google Scholar] [CrossRef]
- Russel, K.R.; Press, B.J.; Rampulla, A.R. Thiophene system 9, thienopyrimidinedione derivatives as potential antihypertensive agent. J. Med. Chem. 1988, 31, 1786–1789. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kumar, S.; Sing, I.; Saxena, K.K.; Kumar, A. Synthesis, characterization and biological activity of various substituted benzothiazol derivatives. Dig. J. Nanomater. Bios. 2010, 5, 67–76. [Google Scholar]
- Badar, S.M.I. Synthesis and anti-inflammatory activity of novel 2,5-disubstituted thiophene derivatives. Turk. J. Chem. 2001, 35, 131–143. [Google Scholar]
- Gronowitz, S.; Raznikiewicz, T. 3-Bromothiophene. Org. Synth. 1964, 44, 9. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).