9a-Phenyl-2,3,3a,3b,9a,9b-hexahydro-4H-furo[3‘,2’:3,4]cyclobuta- [1,2-b]chromen-4-one: A Flavone-Based [2 + 2]-Photocycloadduct
Abstract
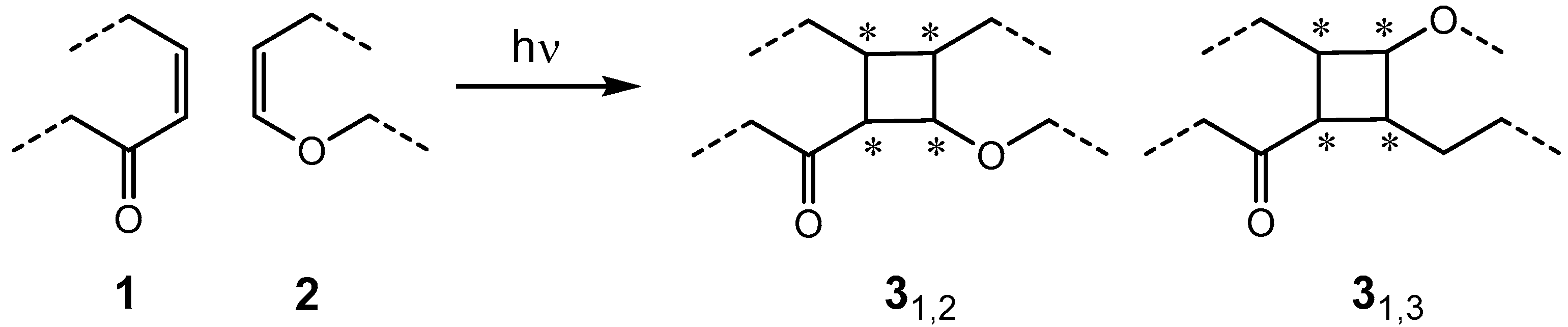
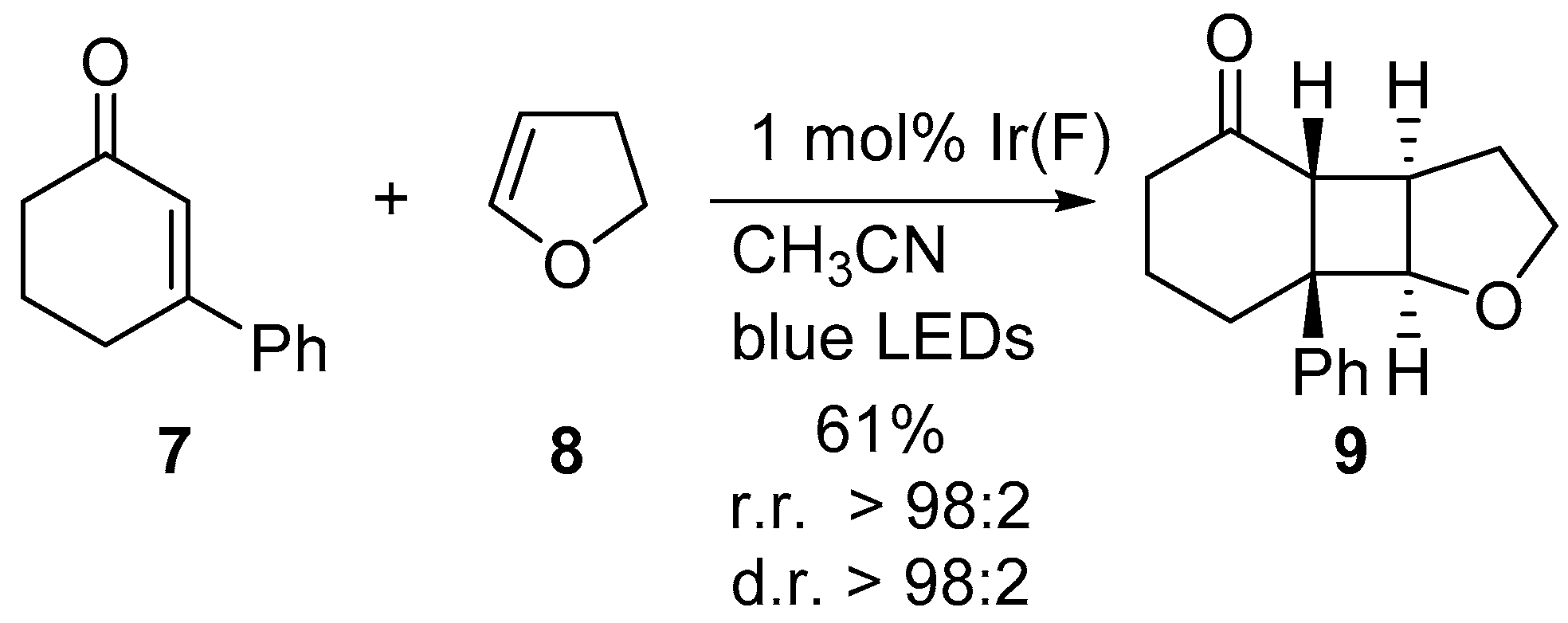
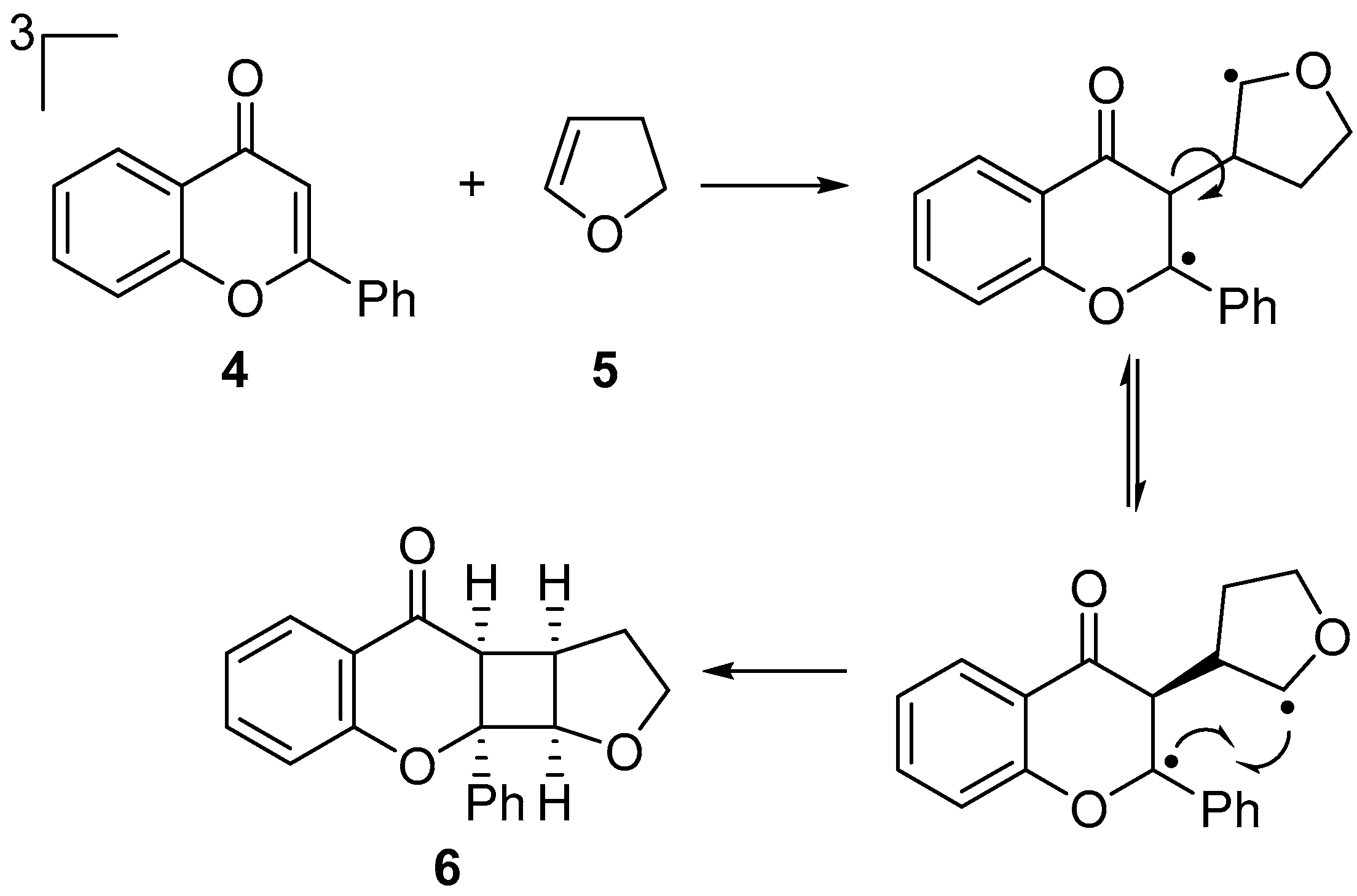
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schuster, D.I.; Lem, G.; Kaprinidis, N.A. New insights into an old mechanism: [2 + 2] photocycloaddition of enones to alkenes. Chem. Rev. 1993, 93, 3–22. [Google Scholar] [CrossRef]
- Bach, T. Stereoselective Intermolecular [2 + 2]-Photocycloaddition Reactions and Their Application in Synthesis. Synthesis 1998, 1998, 683–703. [Google Scholar] [CrossRef]
- Poplata, S.; Tröster, A.; Zou, Y.-Q.; Bach, T. Recent Advances in the Synthesis of Cyclobutanes by Olefin [2 + 2] Photocycloaddition Reactions. Chem. Rev. 2016, 116, 9748–9815. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Bera, N.; Ghosh, S. [2 + 2] Photochemical Cycloaddition in Organic Synthesis. Eur. J. Org. Chem. 2020, 2020, 1310–1326. [Google Scholar] [CrossRef]
- Crimmins, M.T.; Wang, Z.; McKerlie, L.A. Rearrangement of cyclobutyl carbinyl radicals: Total synthesis of the spirovetivane phytoalexin (±)-lubiminol. Tetrahedron Lett. 1996, 37, 8703–8706. [Google Scholar] [CrossRef]
- Mehta, G.; Sreenivas, K. Total synthesis of the novel tricyclic sesquiterpene sulcatine G. Chem. Commun. 2001, 1892–1893. [Google Scholar] [CrossRef]
- Langer, K.; Mattay, J. Stereoselective Intramolecular Copper(I)-Catalyzed [2 + 2]- Photocycloadditions. Enantioselective Synthesis of (+)- and (-)-Grandisol. J. Org. Chem. 2002, 60, 7256–7266. [Google Scholar] [CrossRef]
- Lee-Ruff, E.; Mladenova, G. Enantiomerically Pure Cyclobutane Derivatives and Their Use in Organic Synthesis. Chem. Rev. 2003, 103, 1449–1483. [Google Scholar] [CrossRef]
- Iriondo-Alberdi, J.; Greaney, M.F. Photocycloaddition in Natural Product Synthesis. Eur. J. Org. Chem. 2007, 2007, 4801–4815. [Google Scholar] [CrossRef]
- Hoffmann, N. Photochemical Reactions as Key Steps in Organic Synthesis. Chem. Rev. 2008, 108, 1052–1103. [Google Scholar] [CrossRef]
- Stobbe, H.; Hensel, A. Polymere des Anisal-acetophenons und anderer Chalkone. (I. Mitteilung über Truxill-und Truxin-Ketone). Ber. Dtsch. Chem. Ges. 1926, 59, 2254–2265. [Google Scholar] [CrossRef]
- Corey, E.J.; Bass, J.D.; LeMahieu, R.; Mitra, R.B. A Study of the Photochemical Reactions of 2-Cyclohexenones with Substituted Olefins. J. Am. Chem. Soc. 1964, 86, 5570–5583. [Google Scholar] [CrossRef]
- Cantrell, T.S.; Haller, W.S.; Williams, J.C. Photocycloaddition reactions of some 3-substituted cyclohexenones. J. Org. Chem. 1969, 34, 509–519. [Google Scholar] [CrossRef]
- Sano, T.; Horiguchi, Y.; Tsuda, Y. Dioxopyrrolines. XXXVII Stereochemical pathways of dioxopyrroline-olefin photocycloaddition. Stereochemical selection rule for the photocycloaddition of enone-olefin pairs. Chem. Pharm. Bull. 1987, 35, 23–34. [Google Scholar] [CrossRef][Green Version]
- Lei, T.; Zhou, C.; Huang, M.Y.; Zhao, L.M.; Yang, B.; Ye, C.; Xiao, H.; Meng, Q.Y.; Ramamurthy, V.; Tung, C.H.; et al. General and Efficient Intermolecular [2 + 2] Photodimerization of Chalcones and Cinnamic Acid Derivatives in Solution through Visible-Light Catalysis. Angew. Chem. Int. Ed. 2017, 56, 15407–15410. [Google Scholar] [CrossRef]
- Riener, M.; Nicewicz, D.A. Synthesis of cyclobutane lignans via an organic single electron oxidant–electron relay system. Chem. Sci. 2013, 4, 2625–2629. [Google Scholar] [CrossRef]
- Du, J.; Yoon, T.P. Crossed Intermolecular [2 + 2] Cycloadditions of Acyclic Enones via Visible Light Photocatalysis. J. Am. Chem. Soc. 2009, 131, 14604–14605. [Google Scholar] [CrossRef]
- Oderinde, M.S.; Ramirez, A.; Dhar, T.G.M.; Cornelius, L.A.M.; Jorge, C.; Aulakh, D.; Sandhu, B.; Pawluczyk, J.; Sarjeant, A.A.; Meanwell, N.A.; et al. Photocatalytic Dearomative Intermolecular [2 + 2] Cycloaddition of Heterocycles for Building Molecular Complexity. J. Org. Chem. 2021, 86, 1730–1747. [Google Scholar] [CrossRef]
- Tröster, A.; Alonso, R.; Bauer, A.; Bach, T. Enantioselective Intermolecular [2 + 2] Photocycloaddition Reactions of 2(1H)-Quinolones Induced by Visible Light Irradiation. J. Am. Chem. Soc. 2016, 138, 7808–7811. [Google Scholar] [CrossRef] [PubMed]
- Pecho, F.; Zou, Y.Q.; Gramüller, J.; Mori, T.; Huber, S.M.; Bauer, A.; Gschwind, R.M.; Bach, T. A Thioxanthone Sensitizer with a Chiral Phosphoric Acid Binding Site: Properties and Applications in Visible Light-Mediated Cycloadditions. Chem. Eur. J. 2020, 26, 5190–5194. [Google Scholar] [CrossRef]
- Huang, X.; Quinn, T.R.; Harms, K.; Webster, R.D.; Zhang, L.; Wiest, O.; Meggers, E. Direct Visible-Light-Excited Asymmetric Lewis Acid Catalysis of Intermolecular [2 + 2] Photocycloadditions. J. Am. Chem. Soc. 2017, 139, 9120–9123. [Google Scholar] [CrossRef]
- Du, J.; Skubi, K.L.; Schultz, D.M.; Yoon, T.P. A Dual-Catalysis Approach to Enantioselective [2 + 2] Photocycloadditions Using Visible Light. Science 2014, 344, 392–396. [Google Scholar] [CrossRef]
- Blum, T.R.; Miller, Z.D.; Bates, D.M.; Guzei, I.A.; Yoon, T.P. Enantioselective photochemistry through Lewis acid–catalyzed triplet energy transfer. Science 2016, 354, 1391–1395. [Google Scholar] [CrossRef]
- Lefarth, J. Intermolecular [2 + 2]-Photocycloaddition Reactions of Complex Acceptor-Donor Systems and Development of an Enantioselective Photocatalyzed Hydroformylation Reaction. Ph.D. Thesis, University of Cologne, Köln, Germany, 2021. [Google Scholar]
- Kutateladze, A.G. Conformational Analysis of Singlet−Triplet State Mixing in Paternò−Büchi Diradicals. J. Am. Chem. Soc. 2001, 123, 9279–9282. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5. [Google Scholar] [CrossRef]
- Karak, P. Biological Activities of Flavonoids: An Overview. Int. J. Pharm. Sci. Res. 2019, 3, 1567–1574. [Google Scholar]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Sisa, M.; Bonnet, S.L.; Ferreira, D.; Van der Westhuizen, J.H. Photochemistry of flavonoids. Molecules 2010, 15, 5196–5245. [Google Scholar] [CrossRef]
- Nakayama, T.; Shimizu, T.; Torii, Y.; Miki, S.; Hamanoue, K. A comparison of the photochemistry of flavanone with that of flavone originating from their lowest excited triplet states in ethanol. J. Photochem. Photobiol. A Chem. 1997, 111, 35–39. [Google Scholar] [CrossRef]
- Monici, M.; Mulinacci, N.; Baglioni, P.; Vincieri, F. Flavone photoreactivity. UV-induced reactions in organic solvents and micellar systems. J. Photochem. Photobiol. B Biol. 1993, 20, 167–172. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Paris, C.; Charbonnel, C.; Ghoul, M. The photostability of flavanones, flavonols and flavones and evolution of their antioxidant activity. J. Photochem. Photobiol. A Chem. 2017, 336, 131–139. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Ramaiah, D.; Das, P.K.; George, M.V. A laser flash photolysis study of 2,6-dimethyl-3,5-diphenyl-4-pyrone and related chromones. Evidence for triplet state structural relaxation from quenching behaviors. J. Phys. Chem. 1986, 90, 5984–5989. [Google Scholar] [CrossRef]
- Pownall, H.J. Solvent and substituent effects in aromatic carbonyl compounds: The triplet state of flavone. Spectrochim. Acta Part A Mol. Spectrosc. 1974, 30, 953–959. [Google Scholar] [CrossRef]
- Lowry, M.S.; Goldsmith, J.I.; Slinker, J.D.; Rohl, R.; Pascal, R.A.; Malliaras, G.; Bernhard, S. Single-Layer Electroluminescent Devices and Photoinduced Hydrogen Production from an Ionic Iridium(III) Complex. Chem. Mater. 2005, 17, 5712–5719. [Google Scholar] [CrossRef]
- Engler, G.; Nispel, M.; Marian, C.; Kleinermanns, K. Transient spectroscopy of UV excited flavone: Triplet–triplet absorption and comparison with theory. Chem. Phys. Lett. 2009, 473, 167–170. [Google Scholar] [CrossRef]
- Orlandi, G.; Monti, S.; Barigelletti, F.; Balzani, V. Triplet energy transfer to cis and trans stilbene. A quantum mechanical approach. Chem. Phys. 1980, 52, 313–319. [Google Scholar] [CrossRef]
- Kelly, J.F.D.; Kelly, J.M.; McMurry, T.B.H. Photochemistry of substituted cyclic enones. Part 12. Photocycloaddition of 3-phenylcyclopentenone and 3-phenylcyclohexenone to (E)- and (Z)-1-phenylpropene. J. Chem. Soc. Perkin Trans. 2 1999, 1933–1941. [Google Scholar] [CrossRef]
- Sicignano, M.; Rodríguez, R.I.; Alemán, J. Recent Visible Light and Metal Free Strategies in [2 + 2] and [4 + 2] Photocycloadditions. Eur. J. Org. Chem. 2021, 2021, 3303–3321. [Google Scholar] [CrossRef]
- Data from the Crystal Structure Analysis for 6 is Deposited at the Cambridge Crystallographic Data Centre (CCDC) with the Deposition Number CCDC 2089434. Available online: https://www.ccdc.cam.ac.uk/ (accessed on 21 July 2021).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lefarth, J.; Neudörfl, J.; Griesbeck, A.G. 9a-Phenyl-2,3,3a,3b,9a,9b-hexahydro-4H-furo[3‘,2’:3,4]cyclobuta- [1,2-b]chromen-4-one: A Flavone-Based [2 + 2]-Photocycloadduct. Molbank 2021, 2021, M1256. https://doi.org/10.3390/M1256
Lefarth J, Neudörfl J, Griesbeck AG. 9a-Phenyl-2,3,3a,3b,9a,9b-hexahydro-4H-furo[3‘,2’:3,4]cyclobuta- [1,2-b]chromen-4-one: A Flavone-Based [2 + 2]-Photocycloadduct. Molbank. 2021; 2021(3):M1256. https://doi.org/10.3390/M1256
Chicago/Turabian StyleLefarth, Jens, Jörg Neudörfl, and Axel G. Griesbeck. 2021. "9a-Phenyl-2,3,3a,3b,9a,9b-hexahydro-4H-furo[3‘,2’:3,4]cyclobuta- [1,2-b]chromen-4-one: A Flavone-Based [2 + 2]-Photocycloadduct" Molbank 2021, no. 3: M1256. https://doi.org/10.3390/M1256
APA StyleLefarth, J., Neudörfl, J., & Griesbeck, A. G. (2021). 9a-Phenyl-2,3,3a,3b,9a,9b-hexahydro-4H-furo[3‘,2’:3,4]cyclobuta- [1,2-b]chromen-4-one: A Flavone-Based [2 + 2]-Photocycloadduct. Molbank, 2021(3), M1256. https://doi.org/10.3390/M1256






