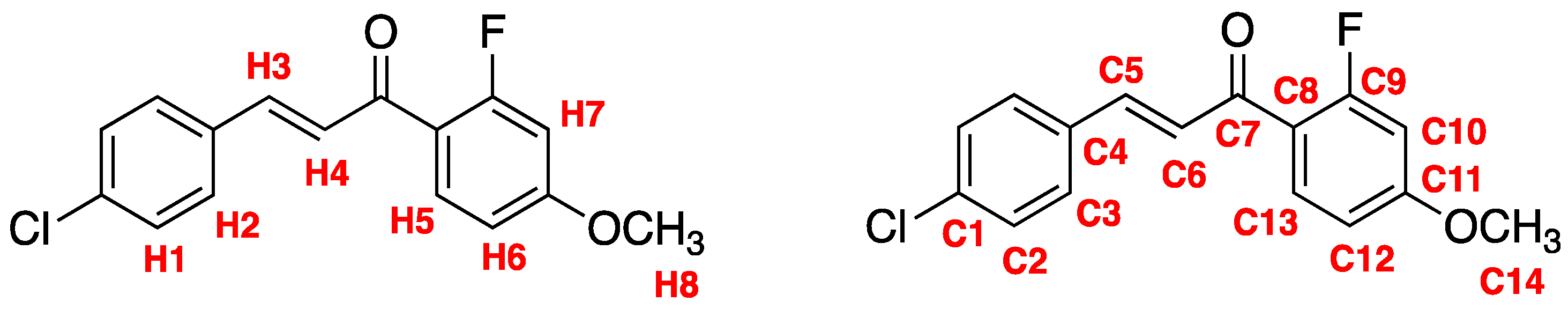
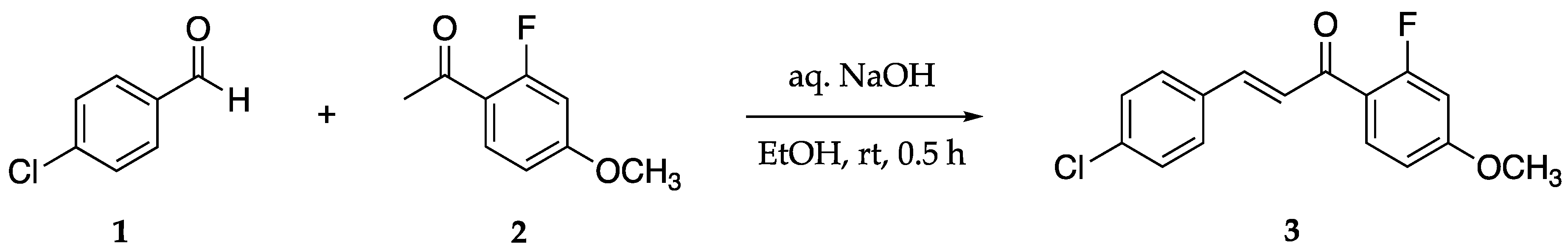Abstract
Natural products known as chalcones show promise as chemotherapeutic agents for the neglected tropical disease known as leishmaniasis. Our objective is to synthesize new targets of opportunity that may lead to better treatments of this debilitating disease. Claisen-Schmidt condensation of 4-chlorobenzaldehyde with 2′-fluoro-4′-methoxyacetophenone using aqueous sodium hydroxide in ethanol yielded the novel compound (E)-3-(4-chlorophenyl)-1-(2-fluoro-4-methoxyphenyl)-2-propen-1-one. The product was obtained in good yield and purity after recrystallization from ethyl acetate/hexane. With the known antiparasitic properties of halogenated chalcones, this novel compound is suitable for antileishmanial activity study.
1. Introduction
Leishmaniasis is the second largest parasitic killer behind malaria. The disease is caused by the flesh-eating protozoan parasite of the genus Leishmania and is transmitted through the bites of phlebotomine sandflies. There are three main forms of leishmaniasis: cutaneous, mucocutaneous, and visceral (kala-azar). Symptoms such as severe skin disfigurement, scarring of the mouth and nose, and organ failure afflict over 1 million people annually [1,2,3].
The World Health Organization classifies leishmaniasis as a neglected tropical disease (NTD), meaning it occurs in tropical or subtropical regions of the Earth. The disease is endemic in Northern Africa, the Middle East, the Mediterranean, parts of Asia, and Central America; impacting those who live in poverty the most. These people typically do not have the access, finances, or ability to obtain treatments, which need intravenous delivery over multiple sessions, thus creating an unfortunate health disparity. The skin lesions and disfiguration caused by the cutaneous and mucosal forms may result in individuals becoming outcasts from their communities and could cause a significant reduction in quality of life.
The areas affected are growing due to vector habitat increase from the warming global climate; with more than 1 billion people in approximately 90 countries at risk of infection due to living in areas where leishmaniasis is endemic. There are no vaccines [4] and current drug treatments suffer from toxicity, high cost, and poor efficacy [5]. Even after treatment, the parasite is not eradicated, it remains dormant in the body to potentially strike again.
The discovery of new effective and affordable medications is imperative to improve the lives of millions worldwide. Fortunately, a group of natural products known as chalcones possess a diverse set of biological activities. These activities include antifungal [6,7], antioxidant [7], and anticancer [8] activity. Recent literature has illustrated the potential for chalcones as a new antileishmanial agent for all three forms of leishmaniasis [9,10,11]. Numerous chalcones reported in literature have exhibited inhibitory activity on promastigotes and/or amastigotes of various Leishmania species [6,7,9,12]. In a study done by Ortalli et al. 31 chalcones were synthesized. Sixteen of them were found to be active against promastigotes of L. donovani. Out of the most promising compounds, one contained a fluorine as well as had low toxicity and high selectivity. Given the potential chalcones have for effectively inhibiting the disease, our objective is to discover new chalcone-based targets of opportunity that may lead to better treatments for leishmaniasis.
2. Results
(E)-3-(4-Chlorophenyl)-1-(2-fluoro-4-methoxyphenyl)-2-propen-1-one 3 was synthesized via a Claisen–Schmidt condensation (Scheme 1). The reaction was performed by adding 4-chlorobenzadehyde 1, 2′-fluoro-4′-methoxyacetonphenone 2, and ethanol to a round bottom flask at room temperature. Aqueous NaOH was added and allowed to stir for 40 min. Optimization of purification methods resulted in the following yields: 78% with recrystallization using ethanol and 85% mixed solvent recrystallization EtOAc/hexanes.
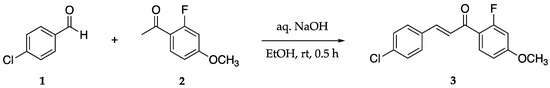
Scheme 1.
Claisen–Schmidt condensation to form chalcone 3.
3. Discussion
The purified compound exhibited the expected spectroscopic signals (1H-NMR, 13C-NMR, IR, and ESI-MS) confirming the successful synthesis of chalcone 3. Using Figure 1 as reference, the 1H-NMR (CDCl3) spectrum shows confirmatory assignments such as the pair of doublets integrating as two hydrogens each for H1 and H2 (J = 8.4 Hz) and the H3 and H4 J value of 15.6 Hz indicating a trans alkene geometry. In addition, both H3 and H4 have a small coupling to fluorine despite the coupling being a 6J and 5J, respectively. This long-range coupling is attributed to the conformation of chalcone 3. Specifically, the most stable conformation brings the fluorine in close spatial proximity to H3 and H4 resulting in the long-range coupling. Positioning of H5, H6, and H7 were corroborated by the 3J values of H5 and H6 to each other (8.7 Hz each) and the 4J of H6 and H7 (2.4 Hz each). All spectra can be found in the Supplementary Materials.
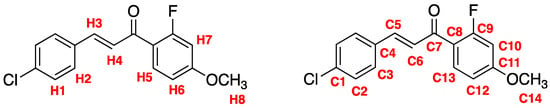
Figure 1.
NMR assignment of chalcone 3.
Notable confirmatory 13C-NMR signals include the α,β-unsaturated carbonyl peak (C7) at 186.9 ppm and the doublet of C9 with a coupling constant of 253 Hz indicative of an aryl fluoride. The vinylic carbons C5 and C6 were delineated by analyzing the shielding/deshielding effects of the various substituents. Such as the aryl chloride, which deshields C5 while also shielding C6. Furthermore, C2 and C3 were differentiated by evaluating the deshielding effects of carbonyl resonance. FT-IR exhibited the following substantiating signals: a sharp α,β unsaturated carbonyl stretch at 1658 cm−1 and a 1587 cm−1 alkene stretch. ESI-MS analysis shows an [M + Na]⁺ ion at 313 m/z, an [M + 1 + Na]⁺ at 314 m/z, an [M + 2 + Na]⁺ ion at 315 m/z, and an [M + 3 + Na]⁺ ion at 316 m/z. The ratio of [M + Na]⁺ to [M + 2 + Na]⁺ peaks are approximately 3:1, which further confirms the structure.
4. Materials and Methods
All chemicals, reagents, and solvents used were obtained from commercial sources (Sigma Aldrich, St. Louis, MO, USA and Fisher Scientific, Waltham, MA, USA) and used without further purification. Thin-layer chromatography (TLC) was used to monitor reactions and performed using aluminum sheets precoated with silica gel 60 (HF254, Merck, Waltham, MA, USA), and visualized with UV radiation (Fisher Scientific, Waltham, MA, USA). The product was characterized by 1H-NMR, 13C-NMR, IR, ESI-MS, and melting point analyses.
IR spectra were recorded on a ThermoFisher iS5 FT-IR (Waltham, MA, USA). Melting point was determined in open capillaries using a Stuart SMP3 melting point apparatus (Cole-Parmer, Vernon Hills, IL, USA). 1H and 13C-NMR spectra were collected using a 500 MHz Bruker AV-500 NMR spectrometer (NuMega Resonance Labs, San Diego, CA, USA). Spectra were referenced to residual CHCl3. Chemical shifts were quoted in ppm and coupling constants (J) were recorded in hertz (Hz). Electrospray ionization (ESI) mass spectrum was acquired using a Perkin Elmer PE-SCIEX API-150 spectrometer (NuMega Resonance Labs, San Diego, CA, USA).
A solution of aqueous NaOH (0.25 mL, 3.75 mmol, 15 M) was added to a round-bottom flask containing 4-chlorobenzaldehyde 1 (0.351 g, 2.50 mmol), 2′-fluoro-4′-methoxyacetophenone 2 (0.420 g, 2.50 mmol), and ethanol (5 mL, anhydrous). The mixture was stirred at room temperature for 30 min (monitored by TLC in 10% EtOAc/hexanes and visualized with UV radiation) during which a yellow-white precipitate formed. The mixture was diluted with water (10 mL), neutralized with 1 M aq. HCl, then cooled to 0 °C, and collected in vacuo, washing twice with ice-cold water (10 mL portions). The crude product was purified by recrystallization from hot ethyl acetate (5 mL) and hexane (10 mL) to yield pure chalcone 3 as light-yellow crystals (0.629 g, 2.13 mmol, 85%) and stored under inert atmosphere.
(E)-3-(4-Chlorophenyl)-1-(2-fluoro-4-methoxyphenyl)-2-propen-1-one (3): mp 98–100 °C; 1H-NMR (CDCl3, 500 MHz): 7.89 ppm (1H, t, JH-H = 8.7 Hz, H5), 7.72 ppm (1H, dd, JH-H = 15.7 Hz, JH-F = 2.0 Hz, H3), 7.55 ppm (2H, d, JH-H = 8.4 Hz, H2), 7.43 ppm (1H, dd, JH-H = 15.6 Hz, JH-F = 2.8 Hz, H4), 7.36 ppm (2H, d, JH-H = 8.4 Hz, H1), 6.79 ppm (1H, dd, JH-H = 8.7 Hz, JH-H = 2.4 Hz, H6), 6.65 ppm (1H, dd, JH-F = 13 Hz, JH-H = 2.4 Hz, H7), 3.87 ppm (3H, s, H8); 13C-NMR (CDCl3, 125 MHz): 186.9 ppm (d, J = 3.7 Hz, C7), 164.8 ppm (d, J = 13 Hz, C11), 163.3 ppm (d, J = 253 Hz, C9) 142.5 ppm (C5), 136.5 ppm (C1), 133.7 ppm (C4), 132.9 ppm (d, J = 3.8 Hz, C13), 129.8 ppm (C3), 129.4 ppm (C2), 126.2 ppm (d, J = 8.8 Hz, C6), 119.6 ppm (d, J = 13 Hz, C8), 111.1 ppm (d, J = 1.25 Hz, C12), 102 ppm (d, J = 28 Hz, C10), 56.1 ppm (C14); FT-IR (KBr) 1658 cm−1 (C=O), 1587 cm−1 (C=C), 809 cm−1 (Ar-Cl); MS (ESI) m/z calc for C16H12O2FCl + Na 313; found 312.9, m/z calc for C16H12O2FCl+H 291; found 291.2.
Supplementary Materials
Copies of the 1H, 13C-NMR, IR, and MS spectra are available online.
Author Contributions
Conceptualization, B.O.A.; methodology, B.O.A.; investigation, B.O.A., A.A. and N.B.; writing—original draft preparation, B.O.A., A.A. and N.B.; writing—review and editing, B.O.A., A.A. and N.B. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by grants from the National Institutes of Health (NIH), National Institute of General Medical Sciences (NIGMS), and IDeA Networks of Biomedical Research Excellence (INBRE), Award number: P20GM103466. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. Leishmaniasis in high-burden countries: An epidemiological update based on data reported in 2014. Wkly. Epidemiol. Rec. 2016, 91, 287–296. [Google Scholar]
- Savoia, D. Recent updates and perspectives on leishmaniasis. J. Infect. Dev. Ctries. 2015, 9, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Pace, D. Leishmaniasis. J. Infect. 2014, 69 (Suppl. 1), S10–S18. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Pecoul, B. “Manifesto” for advancing the control and elimination of neglected tropical diseases. PLoS Negl. Trop. Dis. 2010, 4, e718. [Google Scholar] [CrossRef]
- Barbosa, J.F.; de Figueiredo, S.M.; Monteiro, F.M.; Rocha-Silva, F.; Gaciele-Melo, C.; Coelho, S.S.C.; Lyon, S.; Caligiorne, R.B. New approaches on leishmaniasis treatment and prevention: A review of recent patents. Recent Pat. Endocr. Metab. Immune Drug Discov. 2015, 9, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Zia-ur-Rehman, M.; Zaheer, M.; Ashraf, C.M.; Bolte, M. 1-[4-(1H-Imidazol-1-yl)phenyl]-3-phenylprop-2-en-1-ones—A potential pharmacophore bearing anti-leishmanial activity. J. Chem. Res. 2016, 40, 199–204. [Google Scholar] [CrossRef]
- Hussain, T.; Siddiqui, H.L.; Zia-ur-Rehman, M.; Yasinzai, M.M.; Parvez, M. Anti-oxidant, anti-fungal and anti-leishmanial activities of novel 3-[4-(1H-imidazol-1-yl) phenyl]prop-2-en-1-ones. Eur. J. Med. Chem. 2009, 44, 4654–4660. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, C.; Moorthy, N.S.; Ramasamy, S.; Vanam, U.; Manivannan, E.; Karunagaran, D.; Trivedi, P. Advances in chalcones with anticancer activities. Recent Pat. Anti-Cancer Drug Discov. 2015, 10, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Ortalli, M.; Ilari, A.; Colotti, G.; Ionna, I.D.; Battista, T.; Bisi, A.; Gobbi, S.; Rampa, A.; Di Martino, R.M.C.; Gentilomi, G.A.; et al. Identification of chalcone-based antileishmanial agents targeting trypanothione reductase. Eur. J. Med. Chem. 2018, 152, 527–541. [Google Scholar] [CrossRef] [PubMed]
- de Mello, M.V.P.; Abrahim-Vieira, B.A.; Domingos, T.F.S.; de Jesus, J.B.; de Souza, A.C.C.; Rodrigues, C.R.; de Souza, A.M.T. A comprehensive review of chalcone derivatives as antileishmanial agents. Eur. J. Med. Chem. 2018, 150, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Tajuddeen, N.; Isah, M.B.; Suleiman, M.A.; van Heerden, F.R.; Ibrahim, M.A. The chemotherapeutic potential of chalcones against leishmaniases: A review. Int. J. Antimicrob. Agents. 2018, 51, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Christensen, S.B.; Blom, J.; Lemmich, E.; Nadelmann, L.; Fich, K.; Theander, T.G.; Kharazmi, A.; Licochalcone, A. A Novel Antiparasitic Agent with Potent Activity against Human Pathogenic Protozoan Species of Leishmania. Antimicrob. Agents Chemother. 1993, 37, 2550–2556. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).