Abstract
(2S,3R,6R)-2-[(R)-1-Hydroxyallyl]-4,4-dimethoxy-6-methyltetrahydro-2H-pyran-3-ol was isolated in 18% after treating the glucose derived (5R,6S,7R)-5,6,7-tris[(triethylsilyl)oxy]nona-1,8-dien-4-one with (1S)-(+)-10-camphorsulfonic acid (CSA). The one-pot formation of the title compound involved triethylsilyl (TES) removal, alkene isomerization, intramolecular conjugate addition and ketal formation. The compound was characterized by 1H and 13C NMR spectroscopy, ESI mass spectrometry and IR spectroscopy. NMR spectroscopy was used to establish the product structure, including the conformation of its tetrahydropyran ring.
1. Introduction
Tetrahydropyran rings are found commonly in nature. Pyranoses, for example, are saccharides found in the ‘glycocalyx’, a carbohydrate-rich coating around cells, which is essential for interactions with biomolecules and important in numerous biological processes [1,2], including immunity, inflammation and infection [3,4]. The synthesis of mimetics of pyranoses (glycomimetics) is one strategy being investigated to give new therapies of various diseases, and this approach has received significant attention in recent years [5,6] with many structures investigated being tetrahydropyrans. The scaffolding role of pyranoses has also led to a body of work in the area of new scaffold development for medicinal chemistry [7]. Tetrahydropyrans, which are components of many secondary metabolites, have also provided inspiration for the synthesis of natural product-like compounds, leading to new hits from screening [8]. The synthesis and transformations of tetrahydropyrans has, therefore, relevance to bioactive compound discovery and development.
Herein, a chiral tetrahydropyran with various functionality is prepared from a renewable glucose derivative [9]. The product generated may serve as a scaffold or intermediate in drug discovery or chemical biology projects. The title compound may provide access to saccharide mimics or other valuable analogues of natural products, such as ratjadone, a potent antifungal [10,11].
2. Results and Discussion
The work commenced from methyl α-d-glucopyranoside 1 (Scheme 1). Regioselective iodination of pyranoside 1, followed by O-silylation and subsequent reductive fragmentation with activated zinc dust gave a TES-protected aldehyde [12,13] as a clear oil, using the procedures described previously [14,15,16]. Treatment of this aldehyde with allylmagnesium bromide, followed by oxidation of the resulting alcohol with Dess-Martin periodinane gave ketone 2 as a clear gel-like substance (62%). Acid-catalyzed TES removal from ketone 2 was investigated using camphorsulfonic acid [17]. Treatment of ketone 2 with camphorsulfonic acid in DCM-MeOH at 0 °C to room temperature over 24 h gave the pyran 3 as a yellow gel (18%).
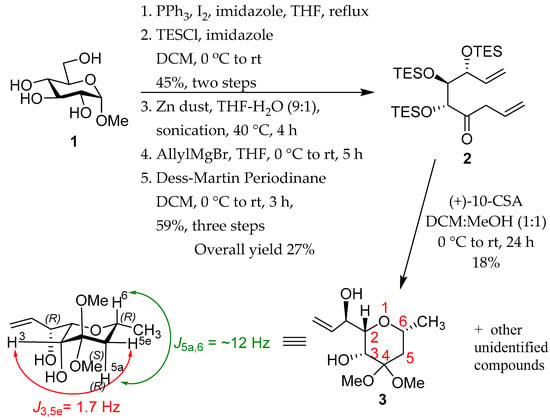
Scheme 1.
Synthetic route to 3.
The tetrahydropyran derivative 3 may arise from a sequence that initially involves the removal of the TES groups, then alkene isomerization via enolisation, to an unsaturated ketone, followed by cyclisation via conjugate addition, and finally ketal formation (see Scheme 2).
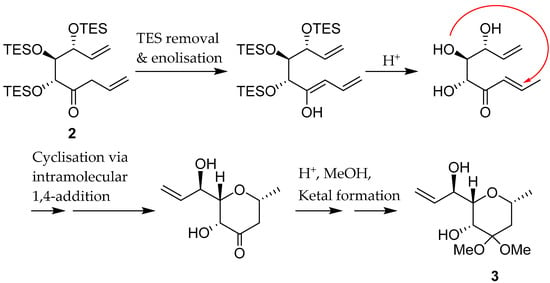
Scheme 2.
Proposal for the formation of 3.
Compound 3 was isolated after chromatography of the product mixture, which contained other as yet unidentified products. To support its structure, 1D and 2D NMR spectroscopy (see Supplementary Materials), IR spectroscopy and ESI mass spectrometry were used. Analysis of the coupling constants in the 1H NMR spectrum, for example, indicated the conformational and configurational assignment shown in Scheme 1. The trans-diaxial arrangement between H-5a and H-6 is supported by the observation of 3J5e.6 value of 11.9 Hz. The 4J3,5e coupling of 1.9 Hz, between H-3 and H-5a, is consistent with a ‘W’ arrangement, which displays 4J values typically <2 Hz [18].
3. Materials and Methods
All analytical data for previously reported compounds was found to be in accordance with data reported in the literature, and citations are provided. All reagents used were obtained from commercial sources and used without further purification. TLC experiments were used to monitor reactions and were performed using aluminium sheets pre-coated with silica gel 60 (HF254, E. Merck, Merck KGaA, Darmstadt, Germany), with spots visualized by UV and charring with cerium (IV) molybdate, vanillin and 5% H2SO4 in MeOH. NMR spectra were processed and analysed using MestReNova software (v14.0.0-23239, https://mestrelab.com, Barcelona, Spain). Chemical shifts were reported relative to internal Me4Si in CDCl3 (δ 0.0) and CD2HOD in CD3OD (δ 3.31) for 1H experiments. CDCl3 (δ 77.16) and CD3OD (δ 49.0) signals were used for 13C experiments. Signals from 1H & 13C spectra were assigned using COSY, HSQC and HMBC. J values are reported as observed. The IR spectra were obtained using a Perkin Elmer Spectrum 100 FTIR Spectrometer. High-resolution mass spectra were obtained using a Waters LCT Premier XE Spectrometer (Etten-Leur, The Netherlands) using positive or negative mode. Chromatography was performed with silica gel 60, using petroleum ether (b.p. 40–60 °C) (PE), EtOAc, DCM and MeOH. DCM, MeOH and THF were used as obtained from a Pure-Solv™ solvent purification system.
(5R,6S,7R)-5,6,7-Tris[(triethylsilyl)oxy]nona-1,8-dien-4-one (2). Methyl α-d-glucopyranoside (1) (6.0 g, 30.9 mmol) was dissolved in THF (40 mL). PPh3 (12.2 g, 46.3 mmol) and imidazole (4.2 g, 61.8 mmol) were added and the reaction mixture was heated to 60 °C. Iodine (11.8 g, 46.3 mmol) was dissolved in THF (5 mL) and added dropwise. The reaction was heated to reflux (75 °C) for 4 h. The reaction was cooled to rt and the salts were filtered. The residue was concentrated to dryness under reduced pressure and column chromatography using gradient elution (95:5 to 90:10 DCM-MeOH) gave the iodo-sugar (6.7 g, 73%, crude) as an orange gel. The iodo-sugar (5.5 g, 18 mmol), which contained traces of triphenylphosphine oxide, was dissolved in DCM (45 mL) under a N2 atmosphere and cooled to 0 °C. Imidazole (7.4 g, 109 mmol) was added to the stirring solution, followed by dropwise addition of TESCl (18.3 mL, 109 mmol). The reaction was left stirring at rt for 3 h. The reaction was quenched with water and the residue was concentrated to dryness under reduced pressure. Column chromatography (elution with PE followed by a gradient from 100:1 to 60:1 PE–EtOAc) gave methyl 6-deoxy-6-iodo-2,3,4-tri-O-triethylsilyl-α-d-glucopyranoside (7.24 g, 62%), which had analytical data in agreement with those previously reported [12]. This intermediate (4.2 g, 6.51 mmol) was dissolved in THF–H2O (90:10, 30 mL) and pre-activated Zn dust (4.3 g, 65.1 mmol) was added. The mixture was sonicated at 40 °C for 4 h. It was then filtered and diluted with Et2O (20 mL). The organic layer was washed with H2O (15 mL × 2), saturated aqueous NaHCO3 (15 mL) and brine (10 mL). The organic layer was dried over Na2SO4 and concentrated to dryness under reduced pressure to give the intermediate aldehyde (3.0 g, 92%, crude) as a clear oil. This compound had analytical data in agreement with those previously reported [12]. This aldehyde was reacted immediately due to risk of decomposition. 1H NMR (500 MHz, CDCl3): δ 9.62 (d, J 0.7 Hz, 1H, aldehyde H, H-1), 5.93 (ddd, J 17.1, 10.4, 7.3 Hz, 1H, H-5), 5.14 (dd, J 17.4, 5.9 Hz, 1H, H-6), 5.09–5.04 (m, 1H, H-6′), 4.31–4.19 (m, 1H, H-4), 3.87 (dd, J 5.5, 1.0 Hz, 1H, H-2), 3.74 (dd, J 6.5, 2.9 Hz, 1H, H-3), 1.05–0.85 (overlapping signals, 27H TES CH3 groups), 0.60–0.46 (overlapping signals, 18H, TES CH2 groups). HRMS (ESI) m/z calc for C24H52O4Si3Na: 511.3071; found 511.3057 (M + Na)+. This aldehyde (3.0 g, 6.22 mmol) was dissolved in dry THF (10 mL) and the solution was stirred for 10 min under a N2 atmosphere at 0 °C. Then, 1 M allylmagnesium bromide in THF (9.3 mL, 9.3 mmol) was then added slowly. The reaction was stirred at 0 °C for 10 min and was then allowed to attain room temperature and stirred for a further 5 h. Satd. aq. NH4Cl solution was added and the mixture was extracted with Et2O (3 × 10 mL). The combined organic layers were washed with brine, dried over Na2SO4. The solvent was removed under reduced pressure to give the intermediate alcohol (3.1 g, 92%) as a clear oil. HRMS (ESI) m/z calc for C27H58O4Si3Na: 553.3541; found 553.3527 (M + Na)+. This alcohol (3.1 g, 5.7 mmol) was dissolved in DCM (15 mL), Dess-Martin periodinane (4.9 g, 11.5 mmol) was added at 0 °C and the mixture was allowed attain room temperature and then stirred for 3 h. The mixture was diluted with Et2O (10 mL) and a 1:1 mixture of NaHCO3–Na2S2O3 (12 mL) was added and stirring was continued for 30 min. The aqueous layer was extracted with Et2O and the combined organic layers were washed with brine, dried over Na2SO4 and the solvent was removed under reduced pressure. Column chromatography (using a gradient mixture of 90:1 to 70:1 PE–EtOAc) gave compound 2 (2.04 g, 68%) as a clear gel like substance. 1H NMR (500 MHz, CDCl3): δ 6.04–5.84 (overlapped signals, 2H, H-2 & H-8), 5.14 (dd, J 17.5, 1.7 Hz, 1H, H-9a), 5.10 (dd, J 10.0, 1.7 Hz, 1H, H-9b), 5.07 (dd, J 9.9, 1.7 Hz, 1H, H-1a), 5.02 (dd, J 17.2, 1.7 Hz, 1H, H-1b), 4.18 (dd, J 6.0, 4.0 Hz, 1H, H-7), 4.04 (d, J 6.3 Hz, 1H, H-5), 3.77 (dd, J 6.3, 4.0 Hz, 1H, H-6), 3.48 (dd, J 18.2, 6.9 Hz, 1H, H-3a), 3.22 (dd, J 18.2, 6.8 Hz, 1H, H-3b), 1.02–0.83 (overlapped signals, 27H, TES -CH3 signals), 0.66–0.46 (overlapped signals, 18H, TES -CH2 signals); 13C NMR (125MHz, CDCl3) δ 206.9 (C-4), 137.5 (C-8), 131.5 (C-2), 117.4 (C-1), 115.8 (C-9), 79.0 (C-5), 77.5 (C-6), 74.5 (C-7), 43.4 (C-3), 6.7 (TES -CH3), 6.6 (TES -CH3), 6.6 (TES -CH3), 5.0 (TES -CH2), 4.8 (TES -CH2), 4.6 (TES -CH2); FTIR (KBr) 2955, 2912, 2878, 1724, 1459, 1415, 1239, 1107, 1087, 1003 cm−1; HRMS (ESI) m/z calc for C27H56O4Si3Na: 551.3384; found 551.3373 (M + Na)+.
(2S,3R,6R)-2-[(R)-1-Hydroxyallyl]-4,4-dimethoxy-6-methyltetrahydro-2H-pyran-3-ol (3). The ketone 2 (1.2 g, 2.27 mmol) was dissolved in 1:1 DCM–MeOH (12 mL) and the mixture was stirred at 0 °C. (+)-10-CSA (2.37 g, 10.2 mmol) was added and the mixture was allowed warm to room temperature and stirred for 12 h. Multiple spots observed on the TLC suggested only partial removal of TES groups from 2 had occurred, so additional (+)-10-CSA (1.84 g, 7.94 mmol) was added and stirred for 12 h, after which a reduced number of spots indicated reaction completion. Column chromatography (using gradient elution from 98:2 to 95:5 DCM–MeOH) gave the title compound 3 (93 mg, 18%) as a yellow gel. 1H NMR (500 MHz, CDCl3): δ 5.87 (ddd, J 17.1, 10.5, 6.7 Hz, 1H, alkene H), 5.42 (dd, J 17.2, 1.5 Hz, 1H, alkene H), 5.23 (dd, J 10.5, 1.5 Hz, 1H, alkene H), 4.34 (t, J 6.7 Hz, 1H, C = HC-CHOH), 3.64 (d, J 1.7 Hz, 1H, H-3), 3.58 (dqd, J 12.3, 6.2, 1.9 Hz, 1H, H-6), 3.39 (d, J 6.7 Hz, 1H, H-2), 3.22 (s, 3H, -OMe), 3.17 (s, 3H, -OMe), 2.96 (s, 1H, -OH), 2.57 (s, 1H, -OH), 1.78 (dt, J 14.0, 1.8 Hz, 1.8 Hz, 1H, H-5e), 1.56 (dd, J 14.0, 11.9 Hz, 1H, H-5a), 1.22 (d, J 6.2 Hz, 3H, -CH3); 13C NMR (125MHz, CDCl3) δ 135.9 (alkene CH), 117.9 (alkene CH2), 99.6 (C-4), 79.0 (C-2), 73.0 (CH2 = CH-CH), 70.4 (C-6), 66.5 (C-3), 47.9 (-OMe), 47.4 (-OMe), 35.2 (C-5), 21.2 (CH3); FTIR (KBr) 3444, 2971, 2936, 2832, 1693, 1588, 1431, 1375, 1316, 1124, 1070, 916 cm−1; HRMS (ESI) m/z calc for C13H23NO5Na 296.1474; found 296.1463 (M + MeCN + Na)+.
Supplementary Materials
The following are available online, 1H and 13C NMR, COSY, HSQC and HMBC spectra, optical rotation data of compounds 2 & 3 are available online.
Author Contributions
Conceptualization, J.B. and P.V.M.; formal analysis, J.B. and P.V.M.; investigation, J.B.; writing—original draft preparation, J.B.; writing—review and editing, J.B. and P.V.M.; supervision, P.V.M.; funding acquisition, J.B. and P.V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors thank the Irish Research Council (grant number EPSPG/2019/440) and Pfizer Ireland, Ringaskiddy, Co. Cork for an Enterprise Partnership PhD study award to J.B. for current funding. We thank the technical staff of the School of Chemistry, NUI Galway for their ongoing support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hevey, R. Strategies for the development of glycomimetic drug candidates. Pharmaceuticals 2019, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Ernst, B.; Magnani, J.L. From carbohydrate leads to glycomimetic drugs. Nat. Rev. Drug Discov. 2009, 8, 661–677. [Google Scholar] [CrossRef]
- Smith, D.C.; Lord, J.M.; Roberts, L.M.; Johannes, L. Glycosphingolipids as toxin receptors. Semin. Cell Dev. Biol. 2004, 15, 397–408. [Google Scholar] [CrossRef]
- Gamblin, S.J.; Skehel, J.J. Influenza hemagglutinin and neuraminidase membrane glycoproteins. J. Biol. Chem. 2010, 285, 28403–28409. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Murphy, P.V.; Gabius, H.J. Multivalent carbohydrate-lectin interactions: How synthetic chemistry enables insights into nanometric recognition. Molecules 2016, 21, 629. [Google Scholar] [CrossRef] [PubMed]
- Tamburrini, A.; Colombo, C.; Bernardi, A. Design and synthesis of glycomimetics: Recent advances. Med. Res. Rev. 2020, 40, 495–531. [Google Scholar] [CrossRef] [PubMed]
- Negi, A.; O’Reilly, C.; Jarikote, D.V.; Zhou, J.; Murphy, P.V. Multi-targeting protein-protein interaction inhibitors: Evolution of macrocyclic ligands with embedded carbohydrates (MECs) to improve selectivity. Eur. J. Med. Chem. 2019, 176, 292–309. [Google Scholar] [CrossRef] [PubMed]
- Voigt, T.; Gerding-Reimers, C.; Ngoc Tran, T.T.; Bergmann, S.; Lachance, H.; Schölermann, B.; Brockmeyer, A.; Janning, P.; Ziegler, S.; Waldmann, H. A natural product inspired tetrahydropyran collection yields mitosis modulators that synergistically target CSE1L and tubulin. Angew. Chem. Int. Ed. 2013, 52, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Kohli, K.; Prajapati, R.; Sharma, B.K. Bio-based chemicals from renewable biomass for integrated biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef]
- Bhatt, U.; Christmann, M.; Quitschalle, M.; Claus, E.; Kalesse, M. The first total synthesis of (+)-ratjadone. J. Org. Chem. 2001, 66, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R.; Ihle, D.C.; Plummer, S.V. Total synthesis of (−)-Ratjadone. Org. Lett. 2001, 3, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Moynihan, L. Synthesis of Iminosugar C-Glycosides via Tandem Allylic-Azide Rearrangement—Huisgen Cycloaddition. Ph.D. Thesis, National University of Ireland, Galway, Ireland, 2012. [Google Scholar]
- Skaanderup, P.R.; Poulsen, C.S.; Hyldtoft, L.; Jørgensen, M.R.; Madsen, R. Regioselective conversion of primary alcohols into iodides in unprotected Methyl furanosides and pyranosides. Synthesis (Stuttg) 2002, 2002, 1721–1727. [Google Scholar] [CrossRef]
- Moynihan, L.; Chadda, R.; McArdle, P.; Murphy, P.V. Allylic azide rearrangement in tandem with huisgen cycloaddition for stereoselective annulation: Synthesis of C-Glycosyl iminosugars. Org. Lett. 2015, 17, 6226–6229. [Google Scholar] [CrossRef] [PubMed]
- Bernet, B.; Vasella, A. Carbocyclische verbindungen aus monosacchariden. II. Umsetzungen in der Mannosereihe. Helv. Chim. Acta 1979, 62, 2400–2410. [Google Scholar] [CrossRef]
- Skaanderup, P.R.; Hyldtoft, L.; Madsen, R. Zinc-mediated fragmentation of methyl 6-deoxy-6-iodo-hexopyranosides. Monatsh. Chem. 2002, 133, 467–472. [Google Scholar] [CrossRef]
- Sasaki, M.; Kawashima, Y.; Fuwa, H. Studies toward the total synthesis of amphidinoliden: Stereocontrolled synthesis of the c13-c29 segment. Heterocycles 2015, 90, 579–599. [Google Scholar] [CrossRef]
- Constantino, M.G.; Lacerda, V.; Tasic, L.; da Silva, G.; Rittner, R. Principal component analysis of long-range ‘W’ coupling constants of some cyclic compounds. J. Mol. Struct. 2001, 597, 129–136. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).