Synthesis of (R) and (S)-3-Chloro-5-(2,4-dimethylpiperazin-1-yl)-4H-1,2,6-thiadiazin-4-ones
Abstract
1. Introduction
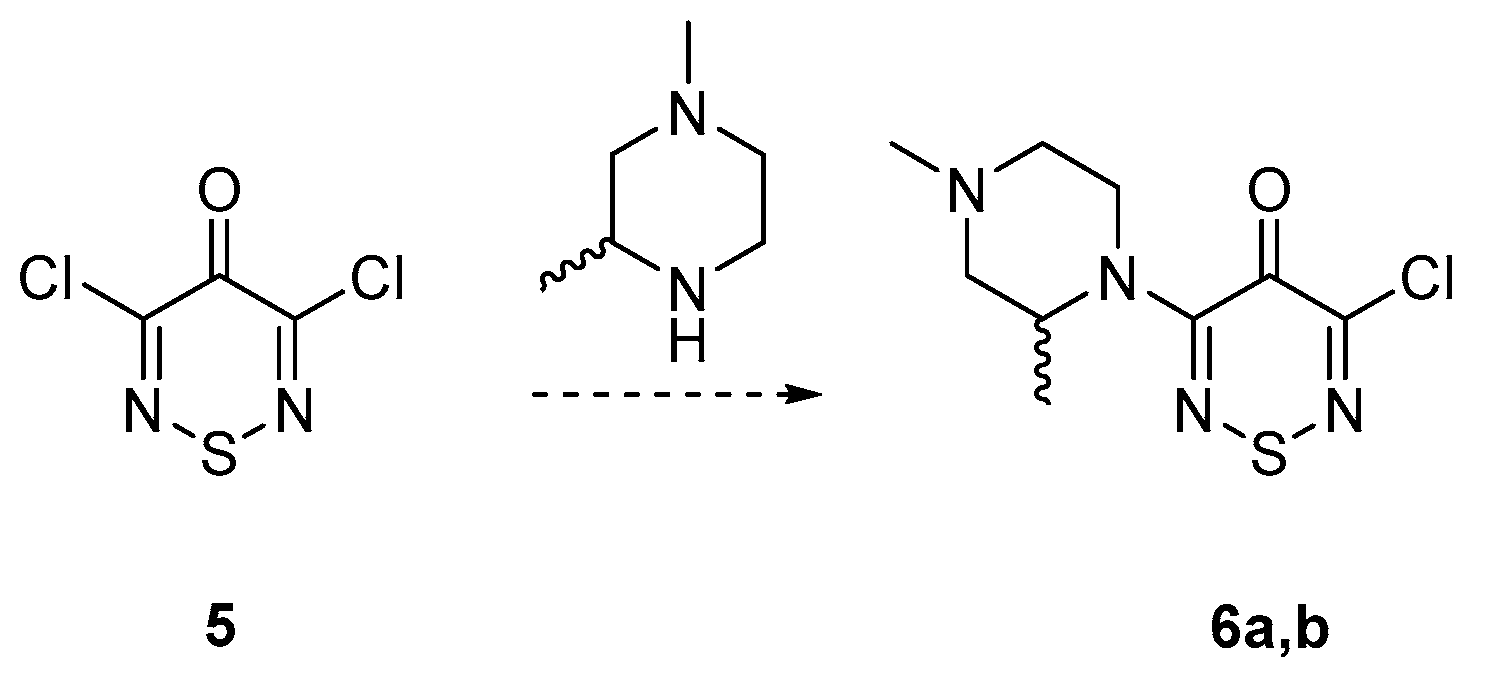
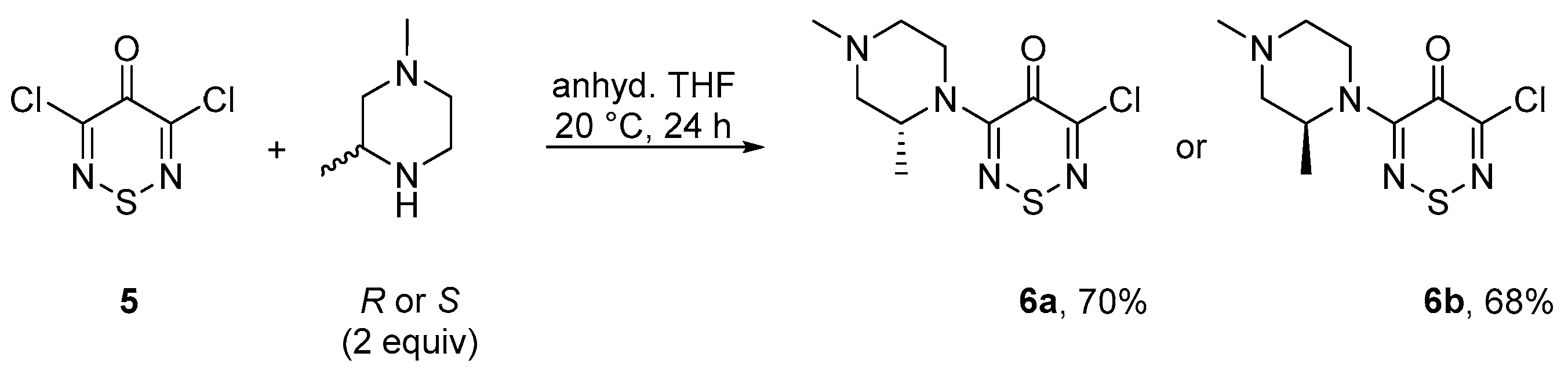
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- James, T.; MacLellan, P.; Burslem, G.M.; Simpson, I.; Grant, J.A.; Warriner, S.; Sridharan, V.; Nelson, A. A modular lead-oriented synthesis of diverse piperazine, 1,4-diazepane and 1,5-diazocane scaffolds. Org. Biomol. Chem. 2014, 12, 2584–2591. [Google Scholar] [CrossRef] [PubMed]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Rochelle, G.; Chen, E.; Freeman, S.; Van Wagener, D.; Xu, Q.; Voice, A. Aqueous piperazine as the new standard for CO2 capture technology. Chem. Eng. 2011, 171, 725–733. [Google Scholar] [CrossRef]
- Boschelli, D.H.; Ye, F.; Wang, Y.D.; Dutia, M.; Johnson, S.L.; Wu, B.; Miller, K.; Powell, D.W.; Yaczko, D.; Young, M.; et al. Optimization of 4-phenylamino-3-quinolinecarbonitriles as potent inhibitors of Src kinase activity. J. Med. Chem. 2001, 44, 3965–3977. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Zhao, F.; Song, H.; Guo, J.; Li, X.; Jiang, X.; Huan, R.; Song, S.; Zhang, Q.; Wang, R.; et al. Structure-based design of 6-chloro-4-aminoquinazoline-2-carboxamide derivatives as potent and selective p21-activated kinase 4 (PAK4) ιinhibitors. J. Med. Chem. 2018, 61, 265–285. [Google Scholar] [CrossRef] [PubMed]
- Islam, I.; Yuan, S.; Wei, R.G.; Xu, W.; Morrissey, M.; Mohan, R.; Zheng, D.; DiMella, A.; Dunning, L.; Snider, M.; et al. Reversible, orally available ADP receptor (P2Y12) antagonists part I: Hit to lead process. Bioorg. Med. Chem. Lett. 2018, 28, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, A.S.; Asquith, C.R.M.; Koutentis, P.A. Synthesis of (R) and (S)-3-Chloro-5-(3-methylmorpholino)-4H-1,2,6-thiadiazin-4-ones. Molbank 2020, 2020, M1128. [Google Scholar] [CrossRef]
- Tan, X.; Tester, R.W.; Luedtke, G.R.; Chakravarty, S.; Mavunkel, B.J.; Perumattam, J.J.; Lu, Q.; Nashashibi, I.; Jung, J.; Hu, J.; et al. Design and synthesis of piperazine-indole p38 alpha MAP kinase inhibitors with improved pharmacokinetic profiles. Bioorg. Med. Chem. Lett. 2010, 20, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Safina, B.S.; Baker, S.; Baumgardner, M.; Blaney, P.M.; Chan, B.K.; Chen, Y.-H.; Cartwright, M.W.; Castanedo, G.; Chabot, C.; Cheguillaume, A.J.; et al. Discovery of novel PI3-kinase δ specific inhibitors for the treatment of rheumatoid arthritis: Taming CYP3A4 time-dependent inhibition. J. Med. Chem. 2012, 55, 5887–5900. [Google Scholar] [CrossRef] [PubMed]
- Talele, T.T. Natural-Products-Inspired Use of the gem-Dimethyl Group in Medicinal Chemistry. J. Med. Chem. 2018, 61, 2166–2210. [Google Scholar] [CrossRef] [PubMed]
- Chochos, C.L.; Kalogirou, A.S.; Ye, T.; Tatsi, E.; Katsouras, A.; Zissimou, G.A.; Gregoriou, V.G.; Avgeropoulos, A.; Koutentis, P.A. 4H-1,2,6-Thiadiazine-containing donor–acceptor conjugated polymers: Synthesis, optoelectronic characterization and their use in organic solar cells. J. Mater. Chem. C 2018, 6, 3658–3667. [Google Scholar] [CrossRef]
- Gómez, T.; Macho, S.; Miguel, D.; Neo, A.G.; Rodríguez, T.; Torroba, T. Cyclopentathiadiazines, cyclohepta- and cyclopentadithiazoles: New materials and a rich heterocyclic chemistry of cyclic enaminonitriles. Eur. J. Org. Chem. 2005, 2005, 5055–5066. [Google Scholar] [CrossRef]
- Peake, C.J.; Harnish, W.N.; Davidson, B.L. Mono-5-substituted-3-chloro-4H-1,2,6-thiadiazin-4-one antifungal agents. U.S. Patent 4,097,594A, 27 June 1978. [Google Scholar]
- Peake, C.J.; Harnish, W.N.; Davidson, B.L. Mono-5-substituted-thio-3-chloro-4H-1,2,6-thiadiazin-4-one antifungal agents. U.S. Patent 4,100,281A, 27 June 1978. [Google Scholar]
- Peake, C.J.; Harnish, W.N.; Davidson, B.L. 3-Chloro-5-(optionally substituted heterocycloxy)-4H-1,2,6-thiadiazin-4-one antifungal agents. U.S. Patent 4,143,138, 3 March 1979. [Google Scholar]
- Peake, C.J.; Harnish, W.N.; Davidson, B.L. Mono-5-substituted-3-chloro-4H-1,2,6-thiadiazin-4-one antifungal agents. U.S. Patent 4,201,780, 6 May 1980. [Google Scholar]
- Portnoy, R.C. Thiadiazinone plant disease control agents. U.S. Patent 4,497,807A, 5 February 1985. [Google Scholar]
- Asquith, C.R.M.; Godoi, P.H.; Couñago, R.M.; Laitinen, T.; Scott, J.W.; Langendorf, C.G.; Oakhill, J.S.; Drewry, D.H.; Zuercher, W.J.; Koutentis, P.A.; et al. 1,2,6-Thiadiazinones as Novel Narrow Spectrum Calcium/Calmodulin-Dependent Protein Kinase Kinase 2 (CaMKK2) Inhibitors. Molecules 2018, 23, 1221. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, A.S.; Koutentis, P.A. The chemistry of non-S-oxidised 4H-1,2,6-thiadiazines. Targets Heterocycl. Syst. 2018, 22, 82–118. [Google Scholar] [CrossRef]
- Geevers, J.; Trompen, W.P. Synthesis and reactions of 3,5-dichloro-4H-1,2,6-thiadiazin-4-one. Recl. Trav. Chim. Pays Bas 1974, 93, 270–272. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W. Reaction of tetracyanoethylene with SCl2; new molecular rearrangements. J. Chem. Soc. Perkin Trans. 2000, 1, 1089–1094. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Koutentis, P.A. A qualitative comparison of the reactivities of 3,4,4,5-tetrachloro-4H-1,2,6-thiadiazine and 4,5-dichloro-1,2,3-dithiazolium chloride. Molecules 2015, 20, 14576–14594. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalogirou, A.S.; Asquith, C.R.M.; Koutentis, P.A. Synthesis of (R) and (S)-3-Chloro-5-(2,4-dimethylpiperazin-1-yl)-4H-1,2,6-thiadiazin-4-ones. Molbank 2020, 2020, M1139. https://doi.org/10.3390/M1139
Kalogirou AS, Asquith CRM, Koutentis PA. Synthesis of (R) and (S)-3-Chloro-5-(2,4-dimethylpiperazin-1-yl)-4H-1,2,6-thiadiazin-4-ones. Molbank. 2020; 2020(2):M1139. https://doi.org/10.3390/M1139
Chicago/Turabian StyleKalogirou, Andreas S., Christopher R. M. Asquith, and Panayiotis A. Koutentis. 2020. "Synthesis of (R) and (S)-3-Chloro-5-(2,4-dimethylpiperazin-1-yl)-4H-1,2,6-thiadiazin-4-ones" Molbank 2020, no. 2: M1139. https://doi.org/10.3390/M1139
APA StyleKalogirou, A. S., Asquith, C. R. M., & Koutentis, P. A. (2020). Synthesis of (R) and (S)-3-Chloro-5-(2,4-dimethylpiperazin-1-yl)-4H-1,2,6-thiadiazin-4-ones. Molbank, 2020(2), M1139. https://doi.org/10.3390/M1139





