Abstract
Selectfluor (1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate)) substitutes the TEMPO free radical with fluorine on 4,7-dimethoxy-1-methyl-2-{[(2,2,6,6-tetramethylpiperidin-1-yl)oxy]methyl}-1H-benzimidazole to give the title compound in a 77% yield. A mechanism is proposed for the formation of this novel methylene fluoride.
1. Introduction
Traditionally, an alkoxyamine undergoes homolysis thermally to give the reactive carbon-centered radical and the stable free radical, nitroxide [1]. TEMPO-Vis is one of a new class of alkoxyamines that releases reactive quinone methide radicals and the nitroxide, (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) upon exposure to visible-light at room temperature (Figure 1) [2]. Light-insensitive 4,7-dimethoxy-1-methyl-2-{[(2,2,6,6-tetramethylpiperidin-1-yl)oxy]methyl}-1H-benzimidazole 1 is the synthetic precursor to TEMPO-Vis and photoactive bis-alkoxyamines.
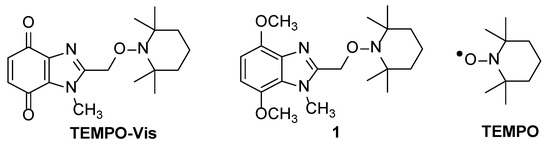
Figure 1.
TEMPO-Vis, synthetic precursor 1, and TEMPO free radical.
Selectfluor (1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate)) is an inexpensive and hazard-free source of electrophilic fluorine [3]. Selectfluor is reported to have fluorinated activated aromatic positions on anisoles [4], phenols [4], naphthols [4,5], and benzamides [5,6]. More recently, the enamine-activated position of benzotriazinones was fluorinated using Selectfluor [7]. As part of our attempts to fluorinate at the activated 4,7-dimethoxybenzene part of alkoxyamine 1 to give 2, an alternative to electrophilic aromatic substitution was discovered, and is now disclosed (Scheme 1).
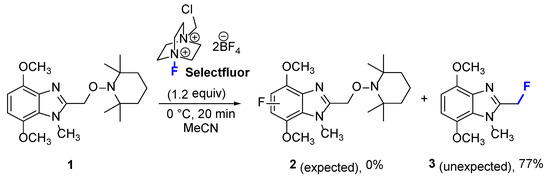
Scheme 1.
Unexpected formation of the title compound 3.
2. Results and Discussion
Treatment of alkoxyamine 1 with Selectfluor (1.2 equiv) at 0 °C led to the rapid liberation of the TEMPO free radical, as indicated by GC-MS (Figure 2), and benzylic fluorination, to give 2-(fluoromethyl)-4,7-dimethoxy-1-methyl-1H-benzimidazole 3. The novel methylene fluoride 3 was isolated in a 77% yield. 2-Fluoromethylbenzimidazole (without the dimethoxy groups) was previously prepared by condensation of 1,2-phenylenediamine with fluoroacetic acid [8]. The expected electrophilic aromatic fluorination at the electron-rich p-dimethoxybenzene part of 1 to give 2 was not observed (Scheme 1).
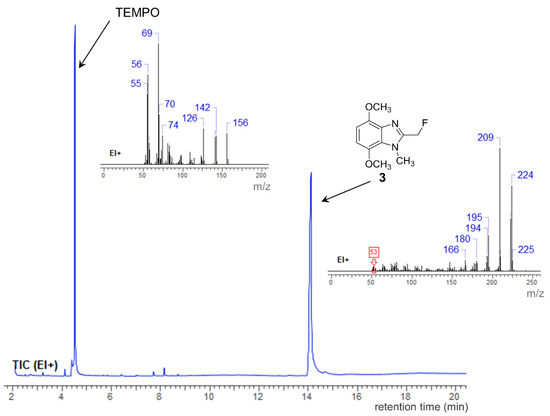
Figure 2.
GC-MS analysis of the reaction mixture for the fluorination of alkoxyamine 1.
The displacement of TEMPO is apparent when comparing the 1H NMR spectra (Figure 3). There are no TEMPO-based peaks in the spectrum of isolated 3, and the methylene signal shifted downfield to 5.61 ppm with splitting into a doublet (2JH-F = 48.2 Hz) due to 1H-19F coupling. The location and multiplicity of the methylene signal is in good agreement with signals reported for 2-(fluoromethyl)-1H-benzimidazole (5.64 ppm, d, 2JH-F = 47.5 Hz) [8].
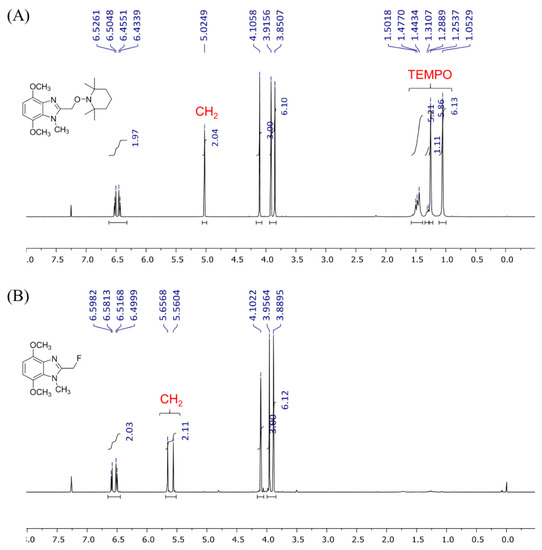
Figure 3.
1H NMR spectra in CDCl3: (A) alkoxyamine 1 and (B) methylene fluoride 3.
The methylene signal split into a doublet (1JC-F = 165.5 Hz) in the 13C NMR spectrum of 3 at 76.8 ppm (see Supplementary Materials for NMR spectra). 13C-19F NMR coupling also gave doublets for benzimidazole-C-2 at 147.1 ppm (2JC-F = 19.0 Hz), and for the N-CH3 at 32.6 ppm (4JC-F = 2.5 Hz). The former is in good agreement with the literature data on 2-(fluoromethyl)-1H-benzimidazole C-2 (148.6 ppm, d, 2JCF = 19.7 Hz) [8].
The 19F NMR signal for 3 at −214.93 ppm is similar to the literature value of −213.92 ppm for 2-(fluoromethyl)-1H-benzimidazole [8]. The signal appeared as a triplet (2JF-H = 48.0 Hz), due to 19F-1H coupling with the two 1H atoms of the adjacent methylene group.
Alkoxyamine 1 is stable to visible-light and the reaction was performed at 0 °C, therefore ruling out bond homolysis as a pathway to formation of methylene fluoride 3. Assuming Selectfluor is a source of F+ or F• and not fluoride, this rules out SN2 displacement of the TEMPO residue [3,9]. Incompatible polarization of the alkoxyamine C–O bond also prevents a simple SH2 mechanism. A single electron transfer (SET) pathway is now proposed, and is supported by the electrochemical oxidations of TEMPO-based alkoxyamines (TEMPO-R) with mesolytic cleavage of the alkoxyamine bond forming TEMPO+ and R• [10]. In this case (Scheme 2), SET is proposed to induce mesolytic cleavage of ‘benzylic alkoxyamine’ 1 to produce TEMPO+ and a methylene radical 4. Abstraction of F• by 4 gives reaction product 3, while reduction of the oxoammonium cation 5 by the Selectfluor-derived DABCO derivative 6 gives the TEMPO free radical detected by GC-MS (Figure 2). A plausible alternative to the mesolytic cleavage is initial SN2 on the fluorine of Selectfluor by the N-3 of benzimidazole 1 to give an imidazolium fluoride [9]. The subsequent generation of 3 eliminates a TEMPO+ species that would undergo reduction by 6 (as in Scheme 2) to give a TEMPO free radical.
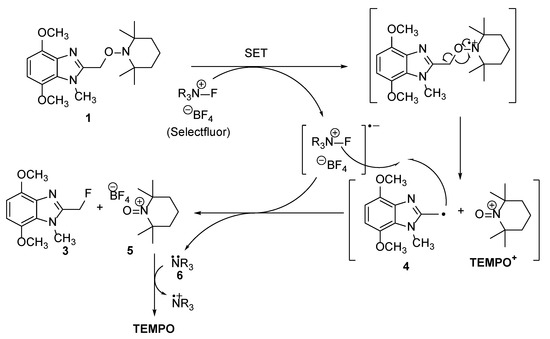
Scheme 2.
Proposed mechanism for the formation of methylene fluoride 3.
3. Materials and Methods
3.1. Materials and Measurements
4,7-Dimethoxy-1-methyl-2-{[(2,2,6,6-tetramethylpiperidin-1-yl)oxy]methyl}-1H-benzimidazole (1) was synthesized in an 83% yield by the base-mediated substitution on 2-(chloromethyl)-4,7-dimethoxy-1-methyl-1H-benzimidazole by TEMPO hydroxylamine (prepared in situ via PtO2 catalyzed hydrogenation of TEMPO (Sigma-Aldrich, 98%, St. Louis, MO, USA) [11]) [2]. 2-(Chloromethyl)-4,7-dimethoxy-1-methyl-1H-benzimidazole was prepared in an 85% overall yield by N-methylation and chlorination of (4,7-dimethoxy-1H-benzimidazol-2-yl)methanol [2,12]. Selectfluor (Sigma-Aldrich, >95% F+ active), MeCN (Sigma-Aldrich, HPLC Plus, ≥99.9%), CH2Cl2 (Fischer Scientific, ≥99%, Hampton, NH, USA) and MgSO4 (Alfa Aesar, 99.5%, Haverhill, MA, USA) were used as received. GC-MS analysis was performed on an Agilent 7890A GC system (Agilent Technologies, Santa Clara, CA, USA), equipped with an Agilent 5975C inert XL Mass Selective Detector (EI) and an RTX-1, 30 m, ID 0.25 mm, FD 0.25 µm column (Restek Corporation, Bellefonte, PA, USA). Helium was used as carrier gas at a flow rate of 0.7 mL/min. The injector was heated to 250 °C, and the oven temperature was increased from 75 to 250 °C at the rate of 10 °C/min, and was then further increased to 350 °C at 50 °C/min. Thin layer chromatography (TLC) was performed on Merck TLC silica gel 60 F254 plates using a UV lamp (254 nm) for visualization. Flash chromatography was performed using silica gel, pore size 60 Å, 230–400 mesh, and particle size 40–63 μm (Sigma-Aldrich) using EtOAc (Fischer Scientific, ≥99%) and hexanes (Fischer Scientific, bp 40–60 °C). The melting point was measured on a Stuart Scientific melting point apparatus, SMP3. Infrared spectrum was recorded using a Perkin-Elmer Spec 1 (Perkin-Elmer, Waltham, MA, USA) with ATR attached. CDCl3 (Sigma-Aldrich, 99.8% atom D + 0.03% Si(CH3)4 v/v) was used as received. NMR spectra were recorded using a Varian 500 MHz instrument (Varian Medical Systems, Palo Alto, CA, USA). The chemical shifts were in ppm relative to Si(CH3)4. NMR assignments were supported by DEPT and 1H-13C correlation. 13C NMR with complete proton decoupling and 19F NMR spectra were collected at 125 and 470 MHz, respectively. HRMS was carried out using ESI time-of-flight mass spectrometer (TOFMS) in positive mode using a Waters LCT Mass Spectrometry instrument (Waters, Milford, MA, USA).
3.2. Synthesis of 2-(Fluoromethyl)-4,7-dimethoxy-1-methyl-1H-benzimidazole (3)
Selectfluor (0.118 g, 0.33 mmol) was added to alkoxyamine 1 (0.100 g, 0.28 mmol) in MeCN (5 mL) at 0 °C and stirred for 20 min. H2O (10 mL) was added and the mixture was extracted with CH2Cl2 (3 × 20 mL). The combined organic layers were dried (MgSO4), evaporated, and the residue purified by flash chromatography using EtOAc and hexanes, as eluent to yield 3 (48 mg, 77%) as a colorless solid; mp 90–92 °C; Rf 0.33 (1:1 EtOAc:hexanes); νmax (neat, cm−1) 3001, 2936, 2838, 1525, 1465, 1392, 1263, 1238, 1221, 1174, 1100, 1070; δH (500 MHz, CDCl3) 3.89 (3H, s, OCH3), 3.96 (3H, s, OCH3), 4.10 (3H, s, NCH3), 5.61 (2H, d, 2JH-F = 48.2 Hz), 6.51 (1H, d, 3JH-H = 8.5 Hz), 6.59 (1H, d, 3JH-H = 8.5 Hz); δC (125 MHz, CDCl3) 32.6 (d, 4JC-F = 2.5 Hz, NCH3), 55.8, 55.9 (both OCH3), 76.8 (d, 1JC-F = 165.5 Hz, CH2), 101.5, 104.0 (both CH), 127.0, 134.2, 141.8, 146.3 (all C), 147.1 (d, 2JC-F = 19.0 Hz, C2); δF (470 MHz, CDCl3) − 214.93 (t, 2JF-H = 48.0 Hz); HRMS (ESI) m/z [M + H]+, C11H14N2O2F calcd. 225.1039, observed 225.1040.
Supplementary Materials
The following are available online: 1H, 13C, and 19F NMR spectra for compound 3.
Author Contributions
P.K. was the only experimentalist, who obtained and analyzed all data. P.K. wrote the manuscript with research director and supervisor, F.A. D.A.S. advised on GC-MS and obtained the research funding with F.A. P.F. became the supervisor of this Ph.D., when F.A. departed NUI Galway for Kingston University. All authors checked and approved the manuscript.
Funding
Irish Research Council Enterprise Partnership Scheme.
Acknowledgments
We are grateful to Peter Cannon for acting as Enterprise Mentor.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Kielty, P.; Farràs, P.; McArdle, P.; Smith, D.A.; Aldabbagh, F. Visible-light unmasking of heterocyclic quinone methide radicals from alkoxyamines. Chem. Commun. 2019, 55, 14665–14668. [Google Scholar] [CrossRef] [PubMed]
- Nyffeler, P.T.; Durόn, S.G.; Burkart, M.D.; Vincent, S.P.; Wong, C.-H. Selectfluor: Mechanistic Insight and applications. Angew. Chem. Int. Ed. 2005, 44, 192–212. [Google Scholar] [CrossRef] [PubMed]
- Banks, R.E.; Besheesh, M.K.; Mohialdin-Khaffaf, S.N.; Sharif, I. N-Halogeno compounds. Part 18. 1-Alkyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane salts: User-friendly site-selective electrophilic fluorinating agents of the N-fluoroammonium class. J. Chem. Soc. Perkin Trans. 1 1996, 16, 2069–2076. [Google Scholar] [CrossRef]
- Heravi, M.R.P. Fluorination of activated aromatic systems with Selectfluor F-TEDA-BF4 in ionic liquids. J. Fluorine Chem. 2008, 129, 217–221. [Google Scholar] [CrossRef]
- Liang, D.; Li, Y.; Gao, S.; Li, R.; Li, X.; Wang, B.; Yang, H. Amide-assisted radical strategy: Metal-free direct fluorination of arenes in aqueous media. Green Chem. 2017, 19, 3344–3349. [Google Scholar] [CrossRef]
- Mirallai, S.I.; Koutentis, P.A.; Aldabbagh, F. Regioselective fluorination of 7-oxo-1,2,4-benzotriazines using Selectfluor. Molecules 2019, 24, 282. [Google Scholar] [CrossRef] [PubMed]
- René, O.; Souverneva, A.; Magnuson, S.R.; Fauber, B.P. Efficient syntheses of 2-fluoroalkylbenzimidazoles and –benzothiazoles. Tetrahedron Lett. 2013, 54, 201–204. [Google Scholar] [CrossRef]
- Liang, T.; Neumann, C.N.; Ritter, T. Introduction of fluorine and fluorine-containing functional groups. Angew. Chem. Int. Ed. 2013, 52, 8214–8264. [Google Scholar] [CrossRef] [PubMed]
- Hammill, C.L.; Noble, B.B.; Norcott, P.L.; Ciampi, S.; Coote, M.L. Effect of Chemical Structure on the Electrochemical Cleavage of Alkoxyamines. J. Phys. Chem. C 2019, 123, 5273–5281. [Google Scholar] [CrossRef]
- Aldabbagh, F.; Busfield, W.K.; Jenkins, I.D.; Thang, S.H. The reactivity of nitroxides towards alkenes. Tetrahedron Lett. 2000, 41, 3673–3676. [Google Scholar] [CrossRef]
- Kielty, P. Heterocyclic Chemistry: Controlled Unmasking of Nitric Oxide and Nitroxides. Ph.D. Thesis, National University of Ireland Galway, Galway City, Ireland, 2019. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).