Abstract
The title compound was obtained in low yield and spectroscopically characterised. Its X-ray structure was compared with the X-ray structures of other crystallographically-characterised 2-unsubstituted 1,3-dithiolanes.
1. Introduction
Some time ago, we reported that strained double bonds, such as that present in norbornene, could interact with the crystalline adduct of tributylphosphine and carbon disulfide to form an equilibrium mixture containing both an ylide form and a zwitterionic phosphonio dithiocarboxylate [1,2,3]. These could be usefully trapped by the addition of an aldehyde or a dipolarophile such as dimethyl acetylenedicarboxylate (DMAD), resulting in either a Wittig reaction [1,2] to give the 2-alkylidene-1,3-dithiolane A or a 1,3-dipolar cycloaddition [3] to give a dihydrotetrathiafulvalene B, respectively (Scheme 1). Later studies revealed a third, less common mode of reaction, where instead of, or in competition with, the formation of A or B, the ylide form is simply hydrolysed to give the 1,3-dithiolane C [4]. The compounds of type C derived from norbornene and norbornadiene were found to have a strong affinity for mercury, and polymers containing this functionality were evaluated for the selective absorption of Hg2+ from aqueous solutions [4].
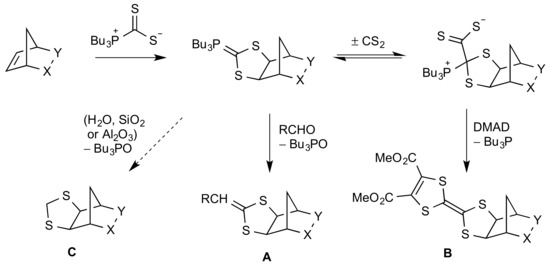
Scheme 1.
Synthetically useful reactions of strained double bonds mediated by Bu3P·CS2.
We describe here the application of conditions designed to produce the product of type B to an oxygen-bridged tricyclic imide substrate that actually produced, in low but significant yield, the product 1 of type C containing a distinctive tetracyclic skeleton. This is characterised spectroscopically, and its structure is confirmed by X-ray diffraction, showing the exo,exo-configuration with the dithiolane ring adopting an envelope conformation.
2. Results
In the hope of generating functionalised dihydrotetrathiafulvalene analogues, we reacted the tricyclic imide 3 with tributylphosphine, carbon disulfide and DMAD (Scheme 2). The imide itself was prepared by treating the readily available furan/maleic anhydride Diels-Alder adduct 2 [5] with butylamine and hexamethyldisilazane [6]. After the reaction, the chromatographic separation of the complex reaction mixture gave one component as a high-melting solid (5.5%) that showed most of the NMR signals expected for starting material 3, but with the disappearance of the alkene signals and the addition of a distinctive AB pattern in the 1H spectrum (δH 3.77 and 4.21, J 10.5 Hz) corresponding to the non-equivalent protons of the SCH2S group. Since there were two very closely similar CH2 signals in the 13C NMR spectrum, an HSQC two-dimensional C–H correlation was required to show that it was the signal at δC 38.7 that was SCH2S while that at δC 39.1 was NCH2 (Supplementary Material, Figure S3). The 1H and 13C NMR and IR spectra were fully in agreement with the structure 1 (see Experimental Section and Supplementary Materials), and this was confirmed by an HRMS measurement in agreement with the molecular formula. Careful examination of the NMR spectra of the crude reaction mixture prior to chromatography showed that compound 1 was not present and it has only been formed by hydrolysis of the intermediate ylide on the silica. This is consistent with our previous work where such hydrolysis was found to be promoted by silica or alumina [4].

Scheme 2.
Synthesis of compound 1.
Since the exo vs. endo configuration of the newly formed dithiolane ring could not be determined with certainty from the NMR data, an X-ray structure determination was performed, and the resulting molecular structure is shown in Figure 1. This clearly shows the exo,exo configuration. The bond lengths and angles are relatively standard (Table 1 and Table 2) and the angle sum at N1 is 360.0(2)°, indicating a completely planar imide nitrogen. The 1,3-dithiolane ring adopts an “envelope” conformation with the plane defined by S6, C7 and S8 at an angle of 40.95° to the mean plane containing S6, C5, C9 and S8. Perhaps surprisingly, the “flap” of the envelope is oriented in the more hindered direction towards the oxygen bridge.
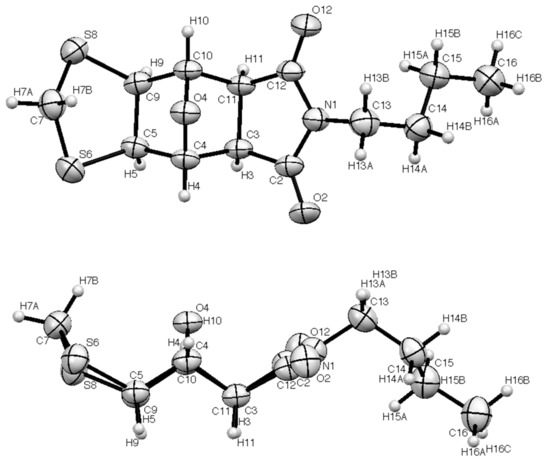
Figure 1.
Two alternative views of the molecular structure of 1 with the numbering scheme used (thermal ellipsoids at 50% level).

Table 1.
Selected bond lengths.

Table 2.
Selected angles.
Examination of the unit cell reveals a centrosymmetric arrangement of four molecules (Figure 2). A search of the Cambridge Structural Database (CSD) yielded a total of only 19 previously determined structures containing a 2-unsubstituted 1,3-dithiolane ring (Figure 3), and, as shown, the vast majority of these are 4,4,5,5-tetrasubstitued since they arise from the 1,3-dipolar cycloaddition of thioketone S-methylides to thioketones, both of which have to be highly substituted for stability.
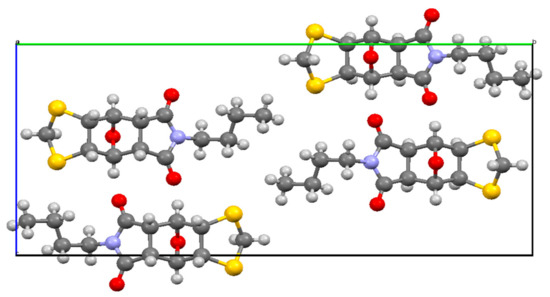
Figure 2.
The unit cell of 1 viewed along the a axis showing the four molecules present in a centrosymmetric arrangement.

Figure 3.
A survey of crystallographically characterised 2-unsubstituted 1,3-dithiolanes with CSD reference codes and literature references [7,8,9,10,11,12,13,14,15,16,17,18,19].
In fact, the only previous disubstituted example is the recently reported 4,4-disubstituted compound 15, and compound 1 seems to the first 4,5-disubstituted 1,3-dithiolane to be crystallographically characterised. It is interesting to note that compound 15 also adopts an envelope conformation with a flap to plane angle of 44° [13], very similar indeed to that observed for 1. The majority of the other tetrasubstituted examples have the dithiolane ring adopting a more twisted shape with no four atoms coplanar. An exception is the rigid polycyclic structure 20, which also exhibits an envelope conformation with a flap to plane angle of 50° [17].
In summary, we obtained the dithiolane-containing tetracyclic imide 1 containing a novel three-dimensional array of functional groups. Its X-ray structure, the first for a 4,5-disubstituted 1,3-dithiolane, shows the exo,exo-configuration, with the dithiolane adopting an envelope conformation.
3. Experimental
Melting points were recorded on a Reichert hot-stage microscope (Reichert, Vienna, Austria) and are uncorrected. IR spectra were recorded on a Perkin-Elmer 1420 instrument (Perkin-Elmer, Waltham, MA, USA). NMR spectra were obtained for protons at 400 MHz and for carbon at 100 MHz using a Bruker AV400 instrument (Bruker, Billerica, MA, USA). Spectra were run at 25 °C on solutions in CDCl3 with internal Me4Si as the reference. Chemical shifts are reported in ppm to high frequency of the reference and the coupling constants J are in Hz.
3.1. 4-Butyl-10-oxa-4-azatricyclo[5.2.1.02,6]dec-8-ene-3,5-dione (3)
Following a literature procedure [6], a solution of 7-oxabicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic anhydride 2 [5] (8.3 g, 50 mmol) in a mixture of methanol (75 mL) and THF (75 mL), was stirred at 0 °C while a solution of butylamine (3.7 g, 50 mmol) in MeOH (12.5 mL) and THF (12.5 mL) was added dropwise. After 30 min, the mixture was heated to 65 °C, and hexamethyldisilane (12.5 mL, 9.7 g, 60 mmol) was added. After reaction at that temperature for 72 h, the mixture was cooled and evaporated under reduced pressure. The residue was taken up in CH2Cl2 (100 mL) and washed successively with aq. NaHCO3, 2 M HCl and water. Drying over MgSO4, followed by evaporation and recrystallisation of the residue from EtOH gave the product (1.65 g, 15%) as colourless crystals, mp 69–70 °C (lit. [20] 74–76 °C). 1H and 13C NMR spectra were in good agreement with Liu et al. [6].
3.2. 10-Butyl-13-oxa-3,5-dithia-10-azatetracyclo[5.5.1.02,6.08,12]tridecane-9,11-dione (1)
A solution of imide 3 (1.50 g, 6.8 mmol), Bu3P·CS2 (1.9 g, 6.8 mmol) and CS2 (0.41 mL, 0.52 g, 6.8 mmol in CH2Cl2 (25 mL)) was stirred at RT for 48 h. Dimethyl acetylenedicarboxylate (0.83 mL, 0.97 g, 6.8 mmol) was added dropwise, resulting in a colour change from orange to dark red. After stirring at RT for 7 days, the mixture was pre-absorbed onto silica gel and subjected to column chromatography (SiO2, CH2Cl2 to 2% MeOH in CH2Cl2). The third of four coloured fractions contained a mixture of unreacted 3 and 1, and this was separated by preparative TLC (SiO2, EtOAc) to give unreacted 3 at Rf 0.4 and the title product (112 mg, 5.5%) recovered from the baseline as colourless crystals, mp 234–236 °C. IR (ATR): 2932, 1771, 1690, 1396, 1344, 1290, 1184, 1136, 964, 887 and 856 cm−1; 1H-NMR (300 MHz, CDCl3): 4.84 (2H, s, CH–O), 4.21 and 3.77 (2H, AB pattern, J 10.5, SCH2S), 4.04 (2H, s, CH–S), 3.45 (2H, t, J 7.4, NCH2), 3.02 (2H, s, CH–C=O), 1.51 (2H, quintet, J 7.5, Bu C-2), 1.27 (2H, sextet, J 7.5, Bu C-3) and 0.90 (3H, t, J 7.2, Bu C-4); 13C-NMR (75 MHz, CDCl3): 175.4 (C=O), 87.6 (CH–O), 60.6 (CH–S), 48.7 (CH–C=O), 39.1 (Bu C-1), 38.7 (SCH2S), 29.5 (Bu C-2), 19.8 (Bu C-3) and 13.6 (Bu C-4); HRMS (ESI): calculated for C13H18NO3S2 (M + H): 300.0728, found: 300.0718; calculated for C13H17NNaO3S2 (M + Na): 322.0548, found: 322.0537.
Crystal data for C13H17NO3S2: M = 299.40 g mol−1, colourless plate, crystal dimensions 0.05 × 0.05 × 0.01 mm, monoclinic, space group P21/n, a = 4.7803(2), b = 25.1943(14), c = 11.4340(6) Å, β = 92.369(5)°, V = 1375.89(12) Å3, Z = 4, Dcalc = 1.445 g cm–3, T = 173 K, R1 = 0.0463, Rw2 = 0.1332 for 2059 reflections with I > 2σ(I), and 173 variables. Data were collected using graphite monochromated Cu Kα radiation λ = 1.54184 Å and have been deposited at the Cambridge Crystallographic Data Centre as CCDC 1989786. The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/getstructures. The structure was solved by direct methods and refined by full-matrix least-squares against F2 (SHELXL, Version 2018/3 [21]).
Supplementary Materials
The following is available online. Figure S1: 1H NMR spectrum of 1; Figure S2: 13C NMR spectrum of 1; Figure S3: HSQC 2D C–H correlation NMR spectrum of 1; Figure S4: IR spectrum of 1.
Author Contributions
F.M.F. prepared the compound; A.M.Z.S. collected the X-ray data and solved the structure; R.A.A. designed the experiments, analyzed the data, and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aitken, R.A.; Massil, T.; Raut, S.V. Cycloaddition of Bun3P·CS2: Direct One-pot Conversion of Strained Double Bonds to 2-Alkylidene-1,3-dithiolanes. J. Chem. Soc. Chem. Commun. 1994, 2603–2604. [Google Scholar] [CrossRef]
- Aitken, R.A.; Carcas, K.; Hill, L.; Massil, T.; Raut, S.V. Cycloaddition of Bun3P·CS2: Direct One-pot Conversion of Strained Double Bonds to 2-Alkylidene-1,3-dithiolanes. Tetrahedron 1997, 53, 2261–2270. [Google Scholar] [CrossRef]
- Aitken, R.A.; Hill, L.; Lightfoot, P. Direct One Pot Construction of Norbornane-fused Dihydrotetrathiafulvalenes. Tetrahedron Lett. 1997, 38, 7927–7930. [Google Scholar] [CrossRef]
- Aitken, R.A.; Aitken, K.M.; Lambert, S.; Playfair, R.; Wilson, N.J. Synthesis of Norbornane-fused 1,3-Dithiolanes and Evaluation of 1,3-Dithiolane-containing Polymers as Absorbants for Mercury(II) Salts. Heterocycles 2012, 84, 1113–1122. [Google Scholar] [CrossRef]
- Diels, O.; Alder, K. Synthesen in der hydro-aromatischen Reihe, II. Mitteilung: Über Cantharidin. Ber Dtsch. Chem. Ges. 1929, 62, 554–562. [Google Scholar] [CrossRef]
- Liu, M.; van Hensbergen, J.; Burford, R.P.; Lowe, A.B. Thiol-Michael coupling chemistry: Facile access to a library of functional exo-7-oxanorbornenes and their ring-opening metathesis (co)polymerization. Polym. Chem. 2012, 3, 1647–1658. [Google Scholar] [CrossRef]
- Mloston, G.; Huisgen, R.; Polborn, K. Cycloadditions of Adamantanethione S-Methylide to Heteromultiple Bonds. Tetrahedron 1999, 55, 11475–11494. [Google Scholar] [CrossRef]
- Huisgen, R.; Mloston, G.; Giera, H.; Langhals, E.; Polborn, K.; Sustmann, R. Aliphatic Thiocarbonyl Ylides and Thiobenzophenone: Experimental Study of Regiochemistry and Methylene Transfer in Cycloadditions. Eur. J. Org. Chem. 2005, 1519–1531. [Google Scholar] [CrossRef]
- Urbaniak, K.; Mloston, G.; Gulea, M.; Masson, S.; Linder, A.; Heimgartner, H. Thio- and Dithioesters as Dipolarophiles in Reactions with Thiocarbonyl Ylides. Eur. J. Org. Chem. 2005, 1604–1612. [Google Scholar] [CrossRef]
- Mloston, G.; Hamera-Faldyga, R.; Linden, A.; Heimgartner, H. Synthesis of ferrocenyl-substituted 1,3-dithiolanes via [3+2]-cycloadditions of ferrocenyl hetaryl thioketones with thiocarbonyl S-methanides. Beilstein J. Org. Chem. 2016, 12, 1421–1427. [Google Scholar] [CrossRef]
- Mloston, G.; Pipiak, P.; Linden, A.; Heimgartner, H. Studies on the Reactions of Thiocarbonyl S-Methanides with Heteraryl Thioketones. Helv. Chim. Acta 2015, 98, 462–473. [Google Scholar] [CrossRef]
- Egli, D.H.; Linden, A.; Heimgartner, H. 1,5-Dipolar Electrocyclizations in Reactions of α-Thioxo Ketones and α-Thioxo Thioamides with Diazo Compounds. Helv. Chim. Acta 2006, 89, 1910–1926. [Google Scholar] [CrossRef][Green Version]
- Mykhaylychenko, S.S.; Markitanov, Y.N.; Rudenko, T.V.; Rusanov, E.B.; Shermolovich, Y.G. Synthesis of 4-polyfluoroalkyl-1,3-dithiolanes via [3+2] cycloaddition of thiocarbonyl ylide to polyfluoroalkanethioamides. Chem. Heterocycl. Compd. 2019, 55, 189–192. [Google Scholar] [CrossRef]
- Mloston, G.; Linden, A.; Heimgartner, H. Regioselektive 1,3-dipolare Cycloadditionen von Thiocarbonyl-yliden mit 1,3-Thiazol-5(4H)-thionen. Helv. Chim. Acta 1991, 74, 1386–1398. [Google Scholar] [CrossRef]
- Kägi, M.; Linden, A.; Heimgartner, H.; Mloston, G. Umsetzung von 1,3-Thiazol-5(4H)-thionen mit Diazomethan. Helv. Chim. Acta 1993, 76, 1715–1728. [Google Scholar] [CrossRef]
- Leino, R.P.; Luttikhedde, H.J.G.; Nasman, J.H. Dispiro(fluorene-9-4’-[1,3]dithiolane-5’,9”-fluorene). Acta Crystallogr. Sect. C 1995, 51, 1593–1594. [Google Scholar] [CrossRef]
- Kansikas, J.; Sipilä, K. An anthracene dimer with a fused 1,3-dithiole ring at 193 K. Acta Crystallogr. Sect. C 2002, 58, 16–18. [Google Scholar] [CrossRef]
- Korotkov, V.E.; Shibaeva, R.P.; Petrov, M.L.; Rubtsova, I.K. Crystal Structure of 2-(4-Thione-1,3-dithiolane-5-ylidene)-4,5-dimethyl-1,3-diselenole, C8H8S3Se2. Kristallografiya 1990, 35, 1005–1007. [Google Scholar]
- Spitsina, N.G.; Konovalikhin, S.V.; Lobach, A.S.; Shilov, G.V.; D’yachenko, O.A. Synthesis and crystal structure of the molecular complex of fullerene C60 with 2-(4-thiono-1,3-dithiolan-5-ylidene)-4,5-dimethyl-1,3-diselenol (C60·2DTDS). Russ. Chem. Bull. 1999, 48, 2273–2278. [Google Scholar] [CrossRef]
- Clevenger, R.C.; Turnbull, K.D. Synthesis on N-Alkylated Maleimides. Synth. Commun. 2000, 30, 1379–1388. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELXL. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).