Abstract
Synthesis of N′-acetyl-3-methyl-1,6-diphenyl-1H-pyrazolo[3,4-b]pyridine-4-carbohydrazide from the phenyl acetates of 3-acetyl-5-(3-methyl-1,6-diphenyl-1H-pyrazolo[3,4-b]pyridine-4-yl)-2,3-dihydro-1,3,4-oxadiazol-2-yl in alkaline medium and its characterization by spectroscopic methods.
1. Introduction
N′-acetyl and N′-benzoyl hydrazide derivatives containing a heterocycle nucleus have shown antimicrobial and anti-TB activities [1,2]. These compounds are usually synthesized from carbohydrazide derivatives by an addition–elimination reaction with acyl chlorides [3,4]. N′-acetyl and N′-benzoyl hydrazides are also used for the synthesis of 1,3,4-oxadiazole compounds, which can be found in the structure of molecules with reported bioactivity to several targets and related to a wide range of diseases [5,6,7].
On the other hand, N′-acetyl derivatives are often found as the metabolites of the hydrazide group from drugs, such as isoniazid [8,9]. However, the administration of acetylisoniazid leads to diacetylhydrazine (CH3CONHNHCOCH3) as the primary metabolite excreted in which the acyl C–N bond from the pyridine–carboxamide is more susceptible to lysis than is the acyl C–N bond from the alkylamide group [8].
Herein, we describe an unexpected approach to obtain N′-acetylhydrazides from hydrolysis of the 3-acetyl-2,3-dihydro-1,3,4-oxadiazole derivatives of 1,6-diphenyl-1H-pyrazolo[3,4-b]pyridine nucleus and their characterization by spectroscopic methods.
2. Results and Discussion
Hydrolysis methods using sodium acetate are widely used in aromatic acetate deprotection reactions, whereas the ordinarily, stable, aliphatic amides require stronger hydrolysis conditions than the ester motif [10]. In this sense, it could be expected that the mild alkaline and/or no heating conditions could provide the selective hydrolysis of the O-acetyl group from the phenyl acetates of 3-acetyl-5-(3-methyl-1,6-diphenyl-1H-pyrazolo[3,4-b]pyridin-4-yl)-2,3-dihydro-1,3,4-oxadiazol-2-yl.
In fact, if the treatment of compounds 1a–1c with a 4.2 M aqueous NaOAc solution in n-butanol at room temperature did not give any reaction, the exposure to a methanolic/aqueous NaHCO3 solution at room temperature resulted as being effective in the O-acetyl group cleavage [11]. Conversely, both the treatment with a 4.2 M aqueous NaOAc solution in n-butanol under conventional heating or microwave irradiation (Methods A and B, respectively) and with a methanolic KOH (3 equiv.) solution (Method C) yielded the N′-acetylhydrazide derivative 2 (Scheme 1), as a consequence of the 2,3-dihydro-1,3,4-oxadiazole rings’ degradation [12]. The opening of the 2,3-dihydro-1,3,4-oxadiazoles was previously reported under acidic conditions only [13,14].
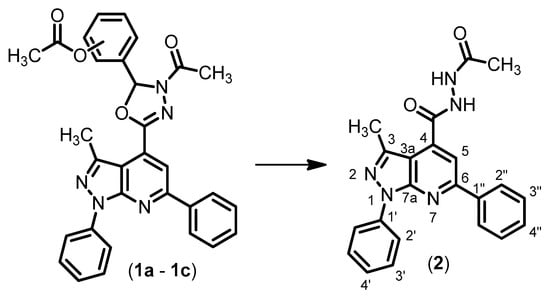
Scheme 1.
N′-acetyl-3-methyl-1,6-diphenyl-1H-pyrazolo[3,4-b]pyridine-4-carbohydrazide (2) from 2,3-dihydro-1,3,4-oxadiazoles derivatives: 1a (4-OAc), 1b (3-OAc-4-OCH3), 1c (3,4-di-OAc). Methods: (A) NaOAc, n-BuOH, reflux; (B) NaOAc, n-BuOH, M.W.; (C) KOH, MeOH, r.t.
The structure of compound 2 was confirmed by spectroscopic methods (Infrared (IR), nuclear magnetic resonance (NMR) and high-resolution mass spectrometry (HRMS), Figures S1–S4). In the protonic nuclear magnetic resonance (1H-NMR) spectrum of compound 2, the signals of the protons from 1,6-dipheny-1H-pyrazolo[3,4-b]pyridine nucleus were observed at δ 2.64 ppm (s, 3H) for the methyl group at the C-3 position; δ 7.36 (dd, 1H), δ 7.55–7.62 ppm (m, 5H), δ 8.25 (d, 2H) and δ 8.33 ppm (d, 2H) for phenyl groups in the N-1 and C-6 positions; and δ 7.90 ppm (s, 1H) for the aromatic proton at the C-5 position [11]. Additionally, one signal at δ 2.00 ppm (s, 3H) corresponding to the methyl group from acetyl-hydrazine moiety and two signals at δ 10.03 ppm (s, 1H) and δ 10.56 ppm (s, 1H) corresponding to hydrazide protons (RCONHNHCOR’), were observed. The protons of the 2,3-dihydro-1,3,4-oxadiazole ring (singlet at δ 7.1 ppm) and of the substituted phenyl groups present in the compounds 1a–1c were not observed [11]. In the same way, the signals corresponding to the carbons of these moieties in the APT spectrum were also not detected. The splitting of signals observed in the NMR spectra of compound 2 could be related to the presence of conformers around the N–CO bond, as reported for similar compounds [15,16]. However, it needs further analysis. In the HRMS spectrum of compound 2, the presence of the molecular ion peak at m/z = 386.1611 ([M + H]+) supported the proposed structure.
In conclusion, we have described the unusual synthesis of N’-acetylhydrazide 2 from derivatives 1a–1c. These results show the lability of the 2,3-dihydro-1,3,4-oxadiazole ring in alkaline medium. This fact could be exploited for stability studies related to drugs containing this moiety.
3. Materials and Methods
All commercial products (Sigma-Aldrich Brasil Ltda., São Paulo, Brazil) and solvents (Vetec Química Fina Ltda., Duque de Caxias, Brazil) were used without further purification. Compounds 1a–1c were synthesized according to methods previously described in the literature [11,17]. The reactions under microwave irradiation were conducted in a domestic microwave device (Brastemp®, São Paulo, Brazil, model BMX35, 900W, 30 L) using a flat bottom balloon connected to an L-shaped adapter joint glassware. All reactions were monitored by thin-layer chromatography (TLC) analysis from reaction aliquots and were performed on Merck 60 F254 aluminum in combination with ultraviolet (UV) detection (254 and 365 nm). Melting points were determined in a capillary Thomas Hoover PC03296 apparatus, and the values are uncorrected. IR spectra were measured on a Varian 660-IR, FT-IR spectrophotometer (Agilent Technologies, Santa Clara, CA, USA), by using the KBr pellet method. NMR spectra were recorded on a Varian VNMRS spectrophotometer (Agilent Technologies, 1H-NMR at 500 MHz and 13C-NMR at 125 MHz).
Proton chemical shifts (δ) are reported in parts per million (ppm) relative to tetramethylsilane (TMS), with the solvent employed as the internal standard (dimethyl sulfoxide DMSO-d6). The structure identification was aided by an Attached Proton Test (APT) spectrum. Mass spectra were recorded on AmaZon SL-Electrospray (ESI) ion trap Bruker spectrometer, and HRMS (positive ESI-Q-TOF) analyses were performed on an Impact HD mass spectrometer (Bruker Corporation, Billerica, MA, USA).
N’-Acetyl-3-methyl-1,6-diphenyl-1H-pyrazolo[3,4-b]pyridine-4-carbohydrazide (2)
Method A
A suspension of 2-(acetoxyphenyl)-2,3-dihydro-1,3,4-oxadiazole (1b, 0.12 g, 0.21 mmol) in n-butanol (1 mL) was stirred at reflux temperature until complete solubilization. Then, NaOAc (0.5 mL of a 4.2 M aqueous solution, 2.1 mmol) was added, and the mixture was stirred for additional 3 h at reflux temperature. The reaction was monitored by TLC using ethyl acetate in hexane 3:4 v/v as the eluent. At the end of the reaction, the solution was cooled at room temperature and poured into water (10 mL). The mixture was extracted with ethyl acetate (3 × 10 mL). The combined organic phases were washed with brine, dried over anhydrous sodium sulfate, and the solvent was removed under vacuum. Recrystallization from methanol gave pure 2 (0.052 g, 64% yield).
Method B
A suspension of 2-(acetoxyphenyl)-2,3-dihydro-1,3,4-oxadiazole (1b or 1c, 0.12 g, 0.21 mmol) in n-butanol (1 mL) was prepared. NaOAc (0.5 mL of a 4.2 M aqueous solution, 2.1 mmol) was added to the mixture and homogenized manually. The mixture was put under microwave irradiation (900 W) at regular intervals of 30 s. The monitoring of the reaction, work-up and purification steps were identical to Method A. The pure compound 2 (0.079 g, 97% yield) was obtained after 3 min or 10 min of reaction from 1b or 1c, respectively.
Method C
KOH (0.016 g, 0.28 mmol) was added to a suspension of 2-(acetoxyphenyl)-2,3-dihydro-1,3,4-oxadiazole (1a, 0.050 g, 0.090 mmol) in methanol (10 mL). The mixture reaction was stirred for 1 h at room temperature, and its progress monitored like previously described. At the end of the reaction, the pH was adjusted to 7.0 (neutral) with a 1M aqueous solution of HCl (20 mL). The mixture was poured into water (10 mL) and extracted with ethyl acetate (3 × 10 mL). The combined organic phases were dried over anhydrous sodium sulfate and the solvent was removed under vacuum. Recrystallization from methanol gave pure 2 (0.032 g; 87% yield).
Structural Characterization
White solid. m.p. = 268–270 °C. Rf = 0.51 (ethyl acetate–hexane, 3:4 v/v). IR (ν-cm−1): 3197 (N–H); 3040 (Csp2–H); 2922 (Csp3–H); 1611 (C=O). 1H-NMR (DMSO-d6/TMS): δ (ppm) = 2.00 (s, 3H, H3C(CO)N–); 2.64 (s, 3H, H-8); 7.36 (t, 1H, J = 7.5 Hz, H-4′); 7.55–7.62 (m, 5H, H-3′/H-3”/H-4″); 7.90 (s, 1H, H-5); 8.25 (d, 2H, J = 7.5 Hz, H-2″); 8.33 (d, 2H, J = 8.0 Hz, H-2′); 10.03 (s, 1H, N-H); 10.56 (s, 1H, N-H). 13C NMR-APT (DMSO-d6/TMS): (ppm) = 14.0 (C-8); 20.2 (H3CC=O); 111.4 (C-3a); 112.7 (C-5); 120.3 (C-2′); 125.4 (C-4′); 127.0 (C-1″); 127.1 (C-2″); 128.7 (C-4″); 128.9 (C-3′); 129.7 (C-3″); 137.7 (C-4); 138.8 (C-1′); 142.2 (C-3); 150.9 (C-7a); 156.0 (C-6); 164.2 (-HNHNC=O); 168.1 (H3CC=O). HRESI-MS m/z 386.1611, ([M + H] + calcd. for C22H19N5O2 386.1611).
Supplementary Materials
The following are available online, Compound 2 spectra: IR (Figure S1); 1H-NMR (Figure S2); APT (Figure S3); HRMS (Figure S4).
Author Contributions
J.C.A.V.S.—synthesis, spectroscopic analysis, writing the manuscript; L.R.S.D.—conceptualization, supervision, data analysis, writing the manuscript. The authors read and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by Brazil’s funding agencies, CAPES (Coordination of Improvement of Higher Education Personnel) and FAPERJ (Foundation for Research Support of the State of Rio de Janeiro).
Acknowledgments
We thank CAPES-BR for the fellowship of J.C.A.V.S.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Azab, M.; El-Hashash, M.; Morsy, J.; Mahmoud, N. Design, synthesis and anticancer activity of novel 2,3- and 2,4-disubstituted quinazoline and quinazolinone derivatives. Heterocycles 2016, 92, 316. [Google Scholar] [CrossRef]
- Joshi, S.D.; Dixit, S.R.; Kulkarni, V.H.; Lherbet, C.; Nadagouda, M.N.; Aminabhavi, T.M. Synthesis, biological evaluation and in silico molecular modeling of pyrrolyl benzohydrazide derivatives as enoyl ACP reductase inhibitors. Eur. J. Med. Chem. 2017, 126, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Pouliot, M.F.; Angers, L.; Hamel, J.D.; Paquin, J.F. Synthesis of 1,3,4-oxadiazoles from 1,2-diacylhydrazines using [Et 2NSF 2]BF 4 as a practical cyclodehydration agent. Org. Biomol. Chem. 2012, 10, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Ali, B.; Khan, K.M.; Iqbal, J.; Ur Rahman, S.; Zaib, S.; Perveen, S. Synthesis and in vitro urease inhibitory activity of benzohydrazide derivatives, in silico and kinetic studies. Bioorg. Chem. 2019, 82, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Boström, J.; Hogner, A.; Llinàs, A.; Wellner, E.; Plowright, A.T. Oxadiazoles in medicinal chemistry. J. Med. Chem. 2012, 55, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Khalilullah, H.; Ahsan, M.J.; Hedaitullah, M.; Khan, S.; Ahmed, B. 1,3,4-oxadiazole: A biologically active scaffold. Mini Rev. Med. Chem. 2012, 12, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Pitasse-Santos, P.; Sueth-Santiago, V.; Lima, M.E.F. 1,2,4- and 1,3,4-oxadiazoles as scaffolds in the development of antiparasitic agents. J. Braz. Chem. Soc. 2018, 29, 435–456. [Google Scholar] [CrossRef]
- Preziosi, P. Isoniazid: Metabolic aspects and toxicological correlates. Curr. Drug Metab. 2007, 8, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Semina, I.I.; Balashov, V.P.; Kurmysheva, T.V.; Tarasova, R.I.; Shilovskaya, E.V.; Voskresenskaya, O.V.; Baichurina, A.Z.; Al’Myasheva, M.I. Synthesis and pharmacological activity of (2-chloroethoxy-4′-dimethylaminophenyl)phosphorylacetic acid hydrazide (CAPAH) and its metabolite (N-acetyl derivative). Pharm. Chem. J. 2013, 47, 28–30. [Google Scholar] [CrossRef]
- Wang, G.B.; Wang, L.F.; Li, C.Z.; Sun, J.; Zhou, G.M.; Yang, D.C. A facile and efficient method for the selective deacylation of N-arylacetamides and 2-chloro-Narylacetamides catalyzed by SOCl2. Res. Chem. Intermed. 2012, 38, 77–89. [Google Scholar] [CrossRef]
- Soares, J.C.A.V. Planejamento, Síntese e Avaliação da Atividade Antichagásica de Novos Derivados 1H-Pirazolo[3,4-b]piridina. Master’s Thesis, Universidade Federal Fluminense, Niterói, Brazil, 2018. [Google Scholar]
- Narender, T.; Reddy, K.P.; Madhur, G. NaOAc-mediated selective deprotection of aromatic acetates and its application in the synthesis of natural products. Synth. Commun. 2009, 39, 1949–1956. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, B.; Di, F.; Xiong, L.; Yang, N.; Li, Y.; Li, Z. Synthesis and biological activities of 2,3-dihydro-1,3,4-oxadiazole compounds and its derivatives as potential activator of ryanodine receptors. Bioorg. Med. Chem. Lett. 2014, 24, 2295–2299. [Google Scholar] [CrossRef] [PubMed]
- Omar, R.H.; El-Fattah, B.A. Synthesis of certain pyridyl 1,3,4-oxadiazoles of biological interest and study of the cleavage of certain substituted oxadiazole rings with primary amines [abstract]. Egypt J. Pharm. Sci. 1985, 24, 49–56. [Google Scholar]
- Hamzi, I.; Fray, M.; Abidi, R.; Barhoumi-Slimi, T. Synthesis, characterization and conformational study of new α,β-unsaturated acylhydrazones based on calix [4]arene backbone. J. Mol. Struct. 2019, 1185, 78–84. [Google Scholar] [CrossRef]
- Litvinov, I.A.; Lodochnikova, O.A.; Bukharov, S.V.; Nugumanova, G.N.; Tagasheva, R.G.; Karamov, F.A. Molecular and crystal structure of indole and camfora with sterically hindered phenol fragments. J. Struct. Chem. 2019, 60, 308–314. [Google Scholar] [CrossRef]
- Lee, L.; Robb, L.M.; Lee, M.; Davis, R.; Mackay, H.; Chavda, S.; Babu, B.; O’Brien, E.L.; Risinger, A.L.; Mooberry, S.L.; et al. Design, synthesis, and biological evaluations of 2,5-diaryl-2,3-dihydro-1,3,4-oxadiazoline analogs of combretastatin-A4. J. Med. Chem. 2010, 53, 325–334. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).