2-Hydroxy-N′-(4-fluorobenzoyl)benzohydrazide
Abstract
1. Introduction
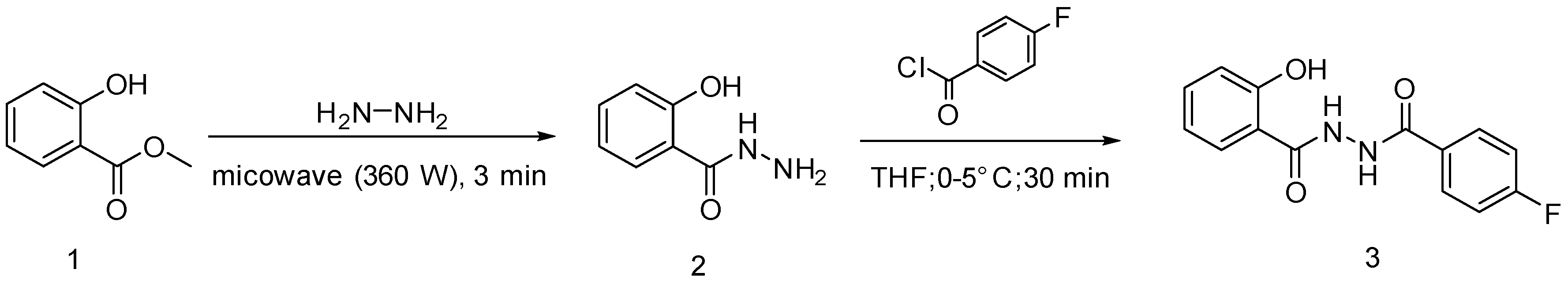
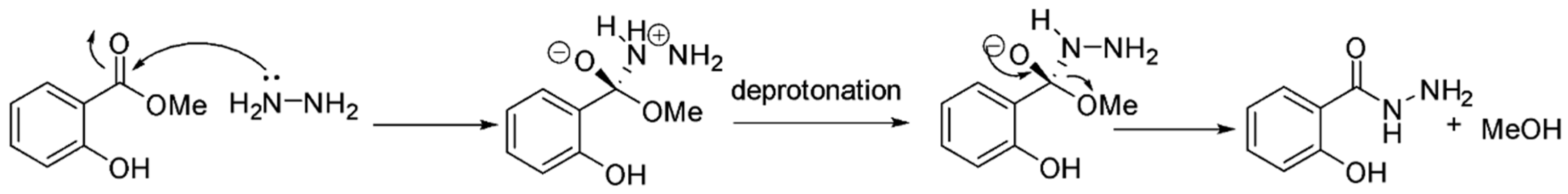
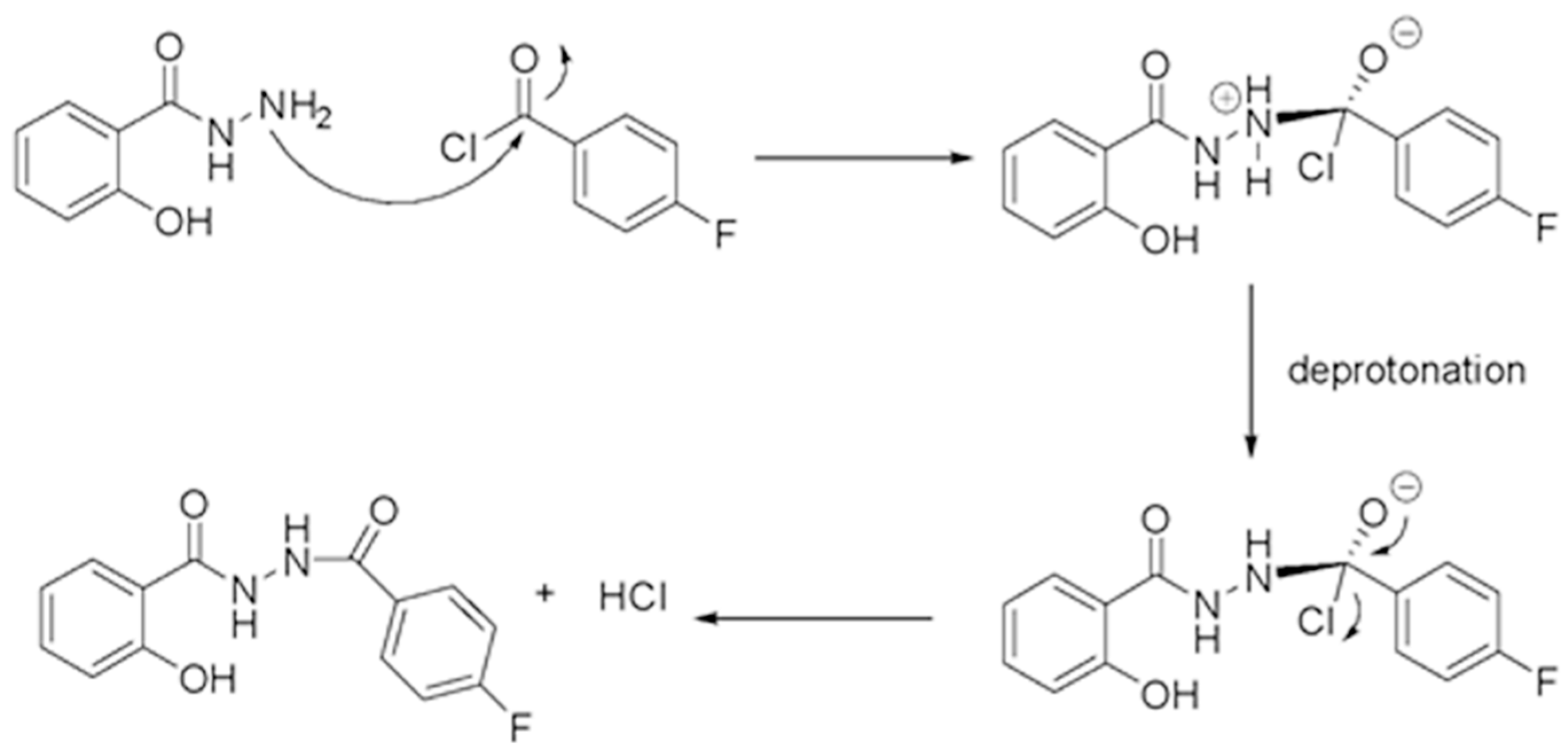
2. Results and Discussion
3. Materials and Methods
3.1. Instruments
3.2. Synthesis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lévesque, H.; Lafont, O. L’aspirine à travers les siècles: Rappels historiques. Rev. Médecine Interne 2000, 21, 8–17. [Google Scholar]
- Wu, K.K. Salicylates and their spectrum of activity. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2007, 6, 278–292. [Google Scholar] [CrossRef]
- Cosmetic Ingredient Review Expert Panel. Safety assessment of salicylic acid, butyloctyl salicylate, calcium salicylate, C12-15 alkyl salicylate, capryloyl salicylic acid, hexyldodecyl salicylate, isocetyl salicylate, isodecyl salicylate, magnesium salicylate, MEA-salicylate, ethylhexyl salicylate, potassium salicylate, methyl salicylate, myristyl salicylate, sodium salicylate, TEA- salicylate, and tridecyl salicylate. Int. J. Toxicol. 2003, 22, 1–108. [Google Scholar]
- Ekinci, D.; Şentürk, M.; Küfevrioğlu, Ö.İ. Salicylic acid derivatives: Synthesis, features, and usage as therapeutic tools. Expert Opin. Ther. Pat. 2011, 21, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Neamati, N.; Hong, H.; Owen, J.M.; Sunder, S.; Winslow, H.E.; Christensen, J.L.; Zhao, H.; Burke, T.R.; Milne, G.W.A.; Pommier, Y. Salicylhydrazine-containing inhibitors of HIV-1 integrase: Implication for a selective chelation in the integrase active site. J. Med. Chem. 1998, 41, 3202–3209. [Google Scholar] [CrossRef]
- Neamati, N.; Lin, Z.; Karki, R.G.; Orr, A.; Cowansage, K.; Strumberg, D.; Pais, G.C.G.; Voigt, J.H.; Nicklaus, M.C.; Winslow, H.E.; et al. Metal-dependent inhibition of HIV-1 integrase. J. Med. Chem. 2002, 45, 5661–5670. [Google Scholar] [CrossRef]
- Al-Mawsawi, L.Q.; Dayam, R.; Taheri, L.; Witvrouw, M.; Debyser, Z.; Neamati, N. Discovery of novel non-cytotoxic salicylhydrazide containing HIV-1 integrase inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 6472–6475. [Google Scholar] [CrossRef]
- Liu, W.R.; Qiao, W.L.; Liu, Z.Z.; Wang, X.H.; Jiang, R.; Li, S.Y.; Shi, R.B.; She, G.M. Gaultheria: Phytochemical and pharmacological characteristics. Molecules 2013, 18, 12071–12108. [Google Scholar] [CrossRef]
- Sadili, A.; Kartawinata, K.; Kartonegoro, A.; Soedjito, H.; Sumadijaya, A. Floristic composition and structure of subalpine summit habitat of Mt. Gede-Pangrango Complex, Cibodas Biosphere Reserve, West Java, Indonesia. Reinwardtia 2009, 12, 393–404. [Google Scholar]
- Santosa, H.; Putra, G.S.; Yuniarta, T.A.; Budiati, T. Synthesis and molecular docking studies of N’-benzoylsalicylhydrazide derivatives as antituberculosis through InhA enzyme inhibition. Indones. J. Pharm. 2018, 29, 198–205. [Google Scholar] [CrossRef]
- Smith, P.A.S. Organic Reactions; Adams, R., Ed.; Wiley: New York, NY, USA, 1946; Chapter 9; Volume 3, pp. 366–369. [Google Scholar]
- Yale, H.L.; Losee, K.; Martins, J.; Holsing, M.; Perry, F.M.; Bernstein, J. Chemotherapy of experimental tuberculosis. VIII. The synthesis of acid hydrazides, their derivatives and related compounds. J. Am. Chem. Soc. 1933, 75, 1933–1942. [Google Scholar] [CrossRef]
- Smith, R.F.; Bates, A.C.; Battisti, A.J.; Byrnes, P.G.; Mroz, C.T.; Smearing, T.J.; Albrecht, F.X. Reactions of hydrazines with esters and carboxylic acids. J. Org. Chem. 1968, 33, 851–855. [Google Scholar] [CrossRef]
- Peng, Y.; Song, G. Simultaneous microwave and ultrasound irradiation: A rapid synthesis of hydrazides. Green Chem. 2001, 3, 302–304. [Google Scholar] [CrossRef]
- Jain, A.K.; Gupta, P.K.; Ganesan, K.; Pande, A.; Malhotra, R.C. Rapid solvent-free synthesis of aromatic hydrazides under microwave irradiation. Def. Sci. J. 2007, 57, 267–270. [Google Scholar] [CrossRef][Green Version]
- Peng, Y.; Song, G. An efficient microwave-assisted one-pot conversion of carboxylic acids into hydrazides. J. Chem. Res. 2003, 12, 768–769. [Google Scholar] [CrossRef]
- Saha, A.; Kumar, R.; Kumar, R.; Devakumar, C. Development and assessment of green synthesis of hydrazides. Indian J. Chem. 2010, 49B, 526–531. [Google Scholar]
- Paulsen, H.; Stoye, D. The Chemistry of Amides; Zabicky, J., Ed.; Interscience: New York, NY, USA, 1970; Chapter 10; pp. 555–557. [Google Scholar]
- Khattab, S.N. Synthesis biological activity of novel amino acid-(N’-benzoyl) hydrazide and amino acid-(N’-nicotinyl) hydrazide derivatives. Molecules 2005, 10, 1218–1228. [Google Scholar] [CrossRef]
- Mashima, M. Infrared absorption spectra of hydrazides. I. Hydrazides of aromatic acids. Bull. Chem. Soc. Jpn. 1962, 35, 332–337. [Google Scholar] [CrossRef]
- El-Baradie, H.Y.F.; Khattab, M.A.; Issa, R.M.; Maghrabi, J.Y. Substituent effects on the spectral behaviour and acid-base properties of arylidene derivatives of salicylic hydrazide. J. Chem. Technol. Biotechnol. 1983, 33A, 123–129. [Google Scholar]
- Weigert, F.J.; Roberts, J.D. 13C nuclear magnetic resonance spectroscopy. Determination of carbon-fluorine couplings. J. Am. Chem. Soc. 1971, 93, 2361–2369. [Google Scholar]
- Muller, N.; Carr, T.D. Carbon-13 splittings in fluorine nuclear magnetic resonance spectra. J. Phys. Chem. 1963, 67, 112–115. [Google Scholar] [CrossRef]
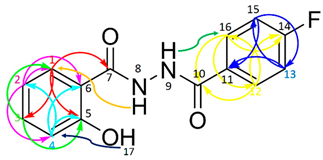 | ||||
| No * | HMQC | COSY/ DPFGSE-NOE | HMBC | |
| H-NMR | C-NMR | |||
| 1 | 7.89 (dd, J = 7.9, 1.7 Hz, 1 H) | 128.9 | H2 | C3; C5; C7 |
| 2 | 6.93 (m, 1 H) | 119.4 | H1; H3 | C1; C4; C6 |
| 3 | 7.43 (ddd, J = 8.3, 7.2, 1.7 Hz, 1 H) | 134.7 | H2; H4 | C1; C5 |
| 4 | 6.95 (m, 1 H) | 117.9 | H3 | C2; C5; C6 |
| 5 | - | 159.8 | - | - |
| 6 | - | 115.2 | - | - |
| 7 | - | 168.2(C = O) | - | - |
| 8 | 10.66(NH) | - | H1 | - |
| 9 | 10.69(NH) | - | H16/H12 | - |
| 10 | - | 165.0 (C = O) | - | - |
| 11 | - | 129.3 (d, J = 3.0 Hz, 1C) | - | - |
| 12 | 7.97 (m, 1 H) | 130.8 (d, J = 10.1 Hz, 1C) | H13 | C10; C14; C16 |
| 13 | 7.34 (m, 1 H) | 116.1 (d, J = 20.2 Hz, 1C) | H12 | C11; C15 |
| 14 | - | 164.8 (d, J = 252.5 Hz, 1C) | - | - |
| 15 | 7.34 (m, 1 H) | 116.1 (d, J = 20.2 Hz, 1C) | H16 | C11; C13 |
| 16 | 7.97 (m, 1 H) | 130.8 (d, J = 10.1 Hz, 1C) | H15 | C10; C12; C14 |
| 17 | 11.88(OH) | - | H4 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santosa, H.; Yuniarta, T.A.; Kesuma, D.; Putra, G.S. 2-Hydroxy-N′-(4-fluorobenzoyl)benzohydrazide. Molbank 2020, 2020, M1103. https://doi.org/10.3390/M1103
Santosa H, Yuniarta TA, Kesuma D, Putra GS. 2-Hydroxy-N′-(4-fluorobenzoyl)benzohydrazide. Molbank. 2020; 2020(1):M1103. https://doi.org/10.3390/M1103
Chicago/Turabian StyleSantosa, Harry, Tegar Achsendo Yuniarta, Dini Kesuma, and Galih Satrio Putra. 2020. "2-Hydroxy-N′-(4-fluorobenzoyl)benzohydrazide" Molbank 2020, no. 1: M1103. https://doi.org/10.3390/M1103
APA StyleSantosa, H., Yuniarta, T. A., Kesuma, D., & Putra, G. S. (2020). 2-Hydroxy-N′-(4-fluorobenzoyl)benzohydrazide. Molbank, 2020(1), M1103. https://doi.org/10.3390/M1103




