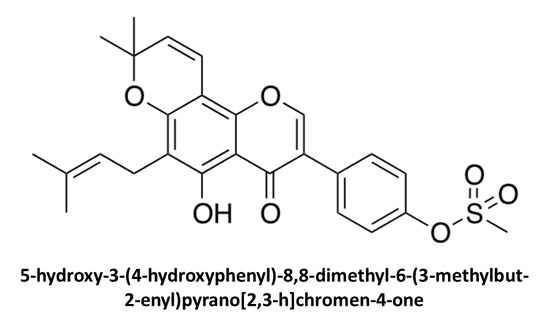
5-Hydroxy-3-(4-hydroxyphenyl)-8,8-dimethyl-6-(3-methylbut-2-enyl)pyrano[2,3-h]chromen-4-one
Abstract
:1. Introduction
2. Results and Discussion
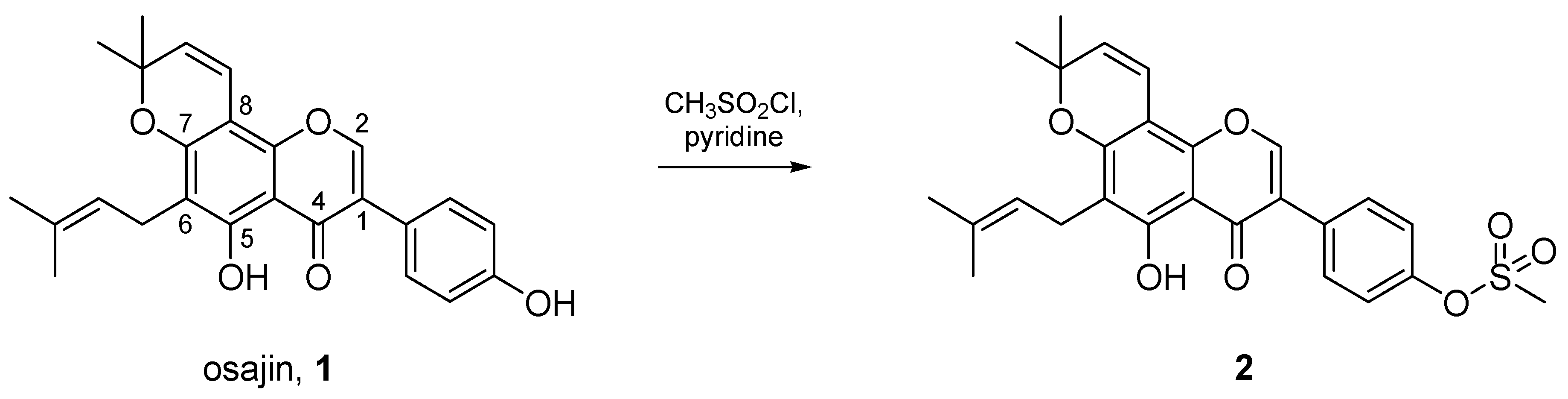
2.1. Extraction of Osajin (1) and Synthesis of 2
2.2. NMR-Based Structural Elucidation and Conformational Studies
2.3. In Silico Studies: Chemical Properties and Preliminary Molecular Docking
3. Materials and Methods
3.1. Chemistry
3.1.1. Extraction of Osajin (1) from M. pomifera
3.1.2. Synthesis of 5-Hydroxy-3-(4-hydroxyphenyl)-8,8-dimethyl-6-(3-methylbut-2-enyl)pyrano[2,3-h]chromen-4-one (2)
3.2. In Silico Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andersson, K.-E. PDE5 Inhibitors—Pharmacology and Clinical Applications 20 Years after Sildenafil Discovery. Br. J. Pharmacol. 2018, 175, 2554–2565. [Google Scholar] [CrossRef] [PubMed]
- Ribaudo, G.; Pagano, M.A.; Bova, S.; Zagotto, G. New Therapeutic Applications of Phosphodiesterase 5 Inhibitors (PDE5-Is). Curr. Med. Chem. 2016, 23, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.-B.; Ma, C.-G.; Liu, T.; Li, W.-R.; Gong, Y.-Q.; Xin, Z.-C. Icarisid II, a PDE5 Inhibitor from Epimedium Wanshanense, Increases Cellular cGMP by Enhancing NOS in Diabetic ED Rats Corpus Cavernosum Tissue. Andrologia 2012, 44, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Pavan, V.; Mucignat-Caretta, C.; Redaelli, M.; Ribaudo, G.; Zagotto, G. The Old Made New: Natural Compounds against Erectile Dysfunction. Arch. Pharm. 2015, 348, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Deng, Y.; Gao, J.; Li, X.; Liu, Y.; Gong, Q. Icariside II, a Novel Phosphodiesterase-5 Inhibitor, Attenuates Streptozotocin-Induced Cognitive Deficits in Rats. Neuroscience 2016, 328, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Du, B.-W.; Lu, D.-F.; Wu, W.-X.; Wongkrajang, K.; Wang, L.; Pu, W.-C.; Liu, C.-L.; Liu, H.-W.; Wang, M.-K.; et al. Flavonoid Glycosides Isolated from Epimedium Brevicornum and Their Estrogen Biosynthesis-Promoting Effects. Sci. Rep. 2017, 7, 7760. [Google Scholar] [CrossRef] [PubMed]
- Adefegha, S.A.; Oboh, G.; Fakunle, B.; Oyeleye, S.I.; Olasehinde, T.A. Quercetin, Rutin, and Their Combinations Modulate Penile Phosphodiesterase-5′, Arginase, Acetylcholinesterase, and Angiotensin-I-Converting Enzyme Activities: A Comparative Study. Comp. Clin. Pathol. 2018, 27, 773–780. [Google Scholar] [CrossRef]
- Ribaudo, G.; Pagano, M.A.; Pavan, V.; Redaelli, M.; Zorzan, M.; Pezzani, R.; Mucignat-Caretta, C.; Vendrame, T.; Bova, S.; Zagotto, G. Semi-Synthetic Derivatives of Natural Isoflavones from Maclura Pomifera as a Novel Class of PDE-5A Inhibitors. Fitoterapia 2015, 105, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Ribaudo, G.; Vendrame, T.; Bova, S. Isoflavones from Maclura Pomifera: Structural Elucidation and in Silico Evaluation of Their Interaction with PDE5. Nat. Prod. Res. 2017, 31, 1988–1994. [Google Scholar] [CrossRef] [PubMed]
- Kupeli, E.; Orhan, I.; Toker, G.; Yesilada, E. Anti-Inflammatory and Antinociceptive Potential of Maclura Pomifera (Rafin.) Schneider Fruit Extracts and Its Major Isoflavonoids, Scandenone and Auriculasin. J. Ethnopharmacol. 2006, 107, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, Z.F. Antimicrobial Components from Maclura Pomifera Fruit. Planta Med. 1981, 42, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R.; Yang, R.; Young, J.C. Antioxidant Isoflavones in Osage Orange, Maclura Pomifera (Raf.) Schneid. J. Agric. Food Chem. 2003, 51, 6445–6451. [Google Scholar] [CrossRef] [PubMed]
- Son, I.H.; Chung, I.-M.; Lee, S.I.; Yang, H.D.; Moon, H.-I. Pomiferin, Histone Deacetylase Inhibitor Isolated from the Fruits of Maclura Pomifera. Bioorg. Med. Chem. Lett. 2007, 17, 4753–4755. [Google Scholar] [CrossRef] [PubMed]
- Orazbekov, Y.; Ibrahim, M.A.; Mombekov, S.; Srivedavyasasri, R.; Datkhayev, U.; Makhatov, B.; Chaurasiya, N.D.; Tekwani, B.L.; Ross, S.A. Isolation and Biological Evaluation of Prenylated Flavonoids from Maclura Pomifera. Evid. Based Complement. Altern. Med. ECAM 2018, 2018, 1370368. [Google Scholar] [CrossRef] [PubMed]
- Wolfrom, M.L.; Benton, F.L.; Gregory, A.S.; Hess, W.W.; Mahan, J.E.; Morgan, P.W. Osage Orange Pigments. II. Isolation of a New Pigment, Pomiferin. J. Am. Chem. Soc. 1939, 61, 2832–2836. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera? A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
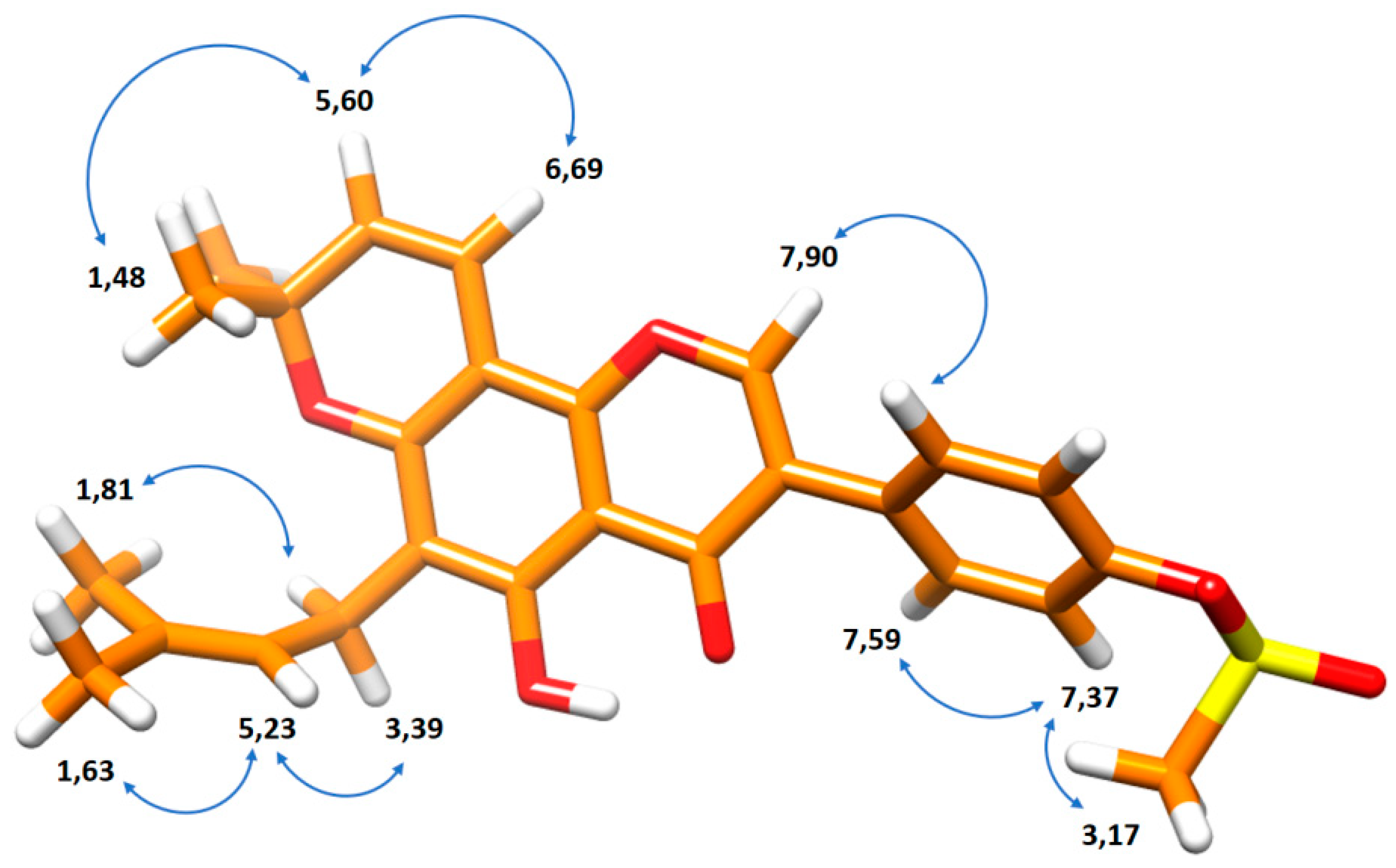
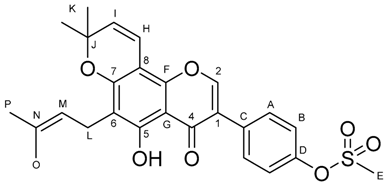
| 1H Chemical Shift (ppm) | 13C Chemical Shift (ppm) | Assignment |
|---|---|---|
| 1.81 | 17.9 | O |
| 3.39 | 21.3 | L |
| 1.63 | 25.8 | P |
| 1.48 | 28.2 | K |
| 3.17 | 37.4 | E |
| - | 78.0 | 8 |
| - | 100.9 | J |
| - | 105.5 | 1 |
| - | 113.2 | G |
| 6.69 | 114.8 | H |
| 5.23 | 121.8 | M |
| - | 122.2 | C |
| 7.37 | 122.5 | B |
| 5.60 | 127.4 | I |
| - | 130.5 | 6 |
| 7.59 | 130.6 | A |
| - | 131.7 | N |
| - | 149.2 | D |
| - | 150.4 | F |
| 7.90 | 152.9 | 2 |
| - | 157.5 | 7 |
| - | 159.3 | 5 |
| - | 180.4 | 4 |
| 13.14 | - | -OH |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribaudo, G.; Ongaro, A.; Zagotto, G. 5-Hydroxy-3-(4-hydroxyphenyl)-8,8-dimethyl-6-(3-methylbut-2-enyl)pyrano[2,3-h]chromen-4-one. Molbank 2018, 2018, M1004. https://doi.org/10.3390/M1004
Ribaudo G, Ongaro A, Zagotto G. 5-Hydroxy-3-(4-hydroxyphenyl)-8,8-dimethyl-6-(3-methylbut-2-enyl)pyrano[2,3-h]chromen-4-one. Molbank. 2018; 2018(3):M1004. https://doi.org/10.3390/M1004
Chicago/Turabian StyleRibaudo, Giovanni, Alberto Ongaro, and Giuseppe Zagotto. 2018. "5-Hydroxy-3-(4-hydroxyphenyl)-8,8-dimethyl-6-(3-methylbut-2-enyl)pyrano[2,3-h]chromen-4-one" Molbank 2018, no. 3: M1004. https://doi.org/10.3390/M1004
APA StyleRibaudo, G., Ongaro, A., & Zagotto, G. (2018). 5-Hydroxy-3-(4-hydroxyphenyl)-8,8-dimethyl-6-(3-methylbut-2-enyl)pyrano[2,3-h]chromen-4-one. Molbank, 2018(3), M1004. https://doi.org/10.3390/M1004