Abstract
The 1-(4-hexylbenzoyl)-3-methylthiourea compound has been successfully synthesized by reacting 4-hexylbenzoyl chloride and 1-methylthiourea via the reflux method using a triethylamine catalyst. The 1-(4-hexylbenzoyl)-3-methylthiourea compound was identified by UV-visible, FT-IR, 13C/1H-NMR and Mass spectrophotometry. From the activity test on four cancer cell lines (HeLa, T47D, WiDr and MCF7 cell), it could be seen that it had better activity on four cancer cells than the control, hydroxyurea.
1. Introduction
Thiourea is an organic compound containing carbon, hydrogen, nitrogen and sulphur atoms. The thiourea structure is similar to the urea, except that the S atom in thiourea replaces the O atom on urea. In previous studies, urea was widely used in drug discovery research, for example as anticancer [1,2], anti-tuberculosis [3,4], antimalarial [5,6] and analgesic [7]. Thiourea was also widely used in new drug discovery research, such as anticancer and antitumor [8,9,10,11,12], antibacterial, antimicrobial and anti-tuberculosis [13,14,15,16,17,18], soluble epoxide hydrolase (sEH) inhibitor [19] and antiviral [20].
In 2015, we synthesized and tested several 1-benzoyl-3-methylthiourea derivatives in HeLa cells [21], and concluded that lipophilic enhancement could increase their activity. From this research, we have designed, synthesized and tested a 1-(4-hexylbenzoyl)-3-methylthiourea compound with much larger lipophilic properties than the previous compounds, as it was predicted to have activity in larger cancer cells. The synthesized 1-(4-hexylbenzoyl)-3-methylthiourea compound was tested against four cancer cell lines, namely: HeLa, MCF7, WiDr, and T47D.
2. Results
2.1. 1-(4-Hexylbenzoyl)-3-methylthiourea
This compound was synthesized by acylation between 4-hexylbenzoyl chloride and N-methyl thiourea, where the nucleophile was N-methylthiourea (Figure 1). The 1-(4-hexylbenzoyl)-3-methylthiourea structure was characterized by the conversion of the -NH2 moiety of N-methylthiourea to -N-C=O (C=O amide). This change could be observed in the 1H-NMR spectra, which showed a proton peak at the -NH region, and was supported by the IR spectra with NH bands.
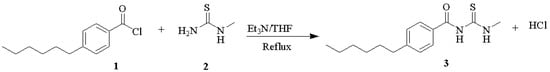
Figure 1.
The scheme of reaction between 4-hexyl benzoyl chloride with N-methylthiourea.
The characteristic υ(N-H) stretching vibration of 1-(4-hexylbenzoyl)-3-methylthiourea appeared at the 3334 cm−1. The strong absorption of the υ(C=O) amide band in the IR spectra of the title compounds was observed at the 1644 cm−1, apparently decreasing in frequency compared with the ordinary carbonyl absorption (1700 cm−1). This is interpreted as being a result of its conjugated resonance with the phenyl ring and the possible formation of intramolecular hydrogen bonding with N-H. The frequency at 1172 cm−1 was assigned to the υ(C=S) vibration for this compound. Besides using the infrared spectrum, the synthesized compound has also been confirmed by 1H/13C-NMR and MS spectrophotometry [22].
2.2. Cytotoxic Activity
The cytotoxic activity of the title compound was initially evaluated against T47D, MCF-7, WiDr, and HeLa cell lines with the MTT assay using hydroxyl urea as a positive control. As listed in Table 1, the IC50 value of 1-(4-hexylbenzoyl)-3-methylthiourea compound was lower than hydroxyurea.

Table 1.
IC50 in vitro cytotoxicity of the tested compounds against HeLa cell line.
Table 1 showed that the 1-(4-hexylbenzoyl)-3-methylthiourea was more potent against T47D, MCF-7, WiDr and HeLa cell lines if it was compared with hydroxyurea. It seems that addition of the 4-hexylbenzoyl and methyl groups to the thiourea structure may increase the potency of the cytotoxic activity. From the data, we concluded that the 1-(4-hexylbenzoyl)-3-methylthiourea compound has the best anticancer activity on T47D cell line (IC50 = 179 μM).
3. Materials and Methods
All reagents and solvents were purchased from standard commercial suppliers. Melting points were measured with an electrothermal melting point apparatus (Cole-Palmer Ltd., Staffordshire, UK) without correction. IR spectra were recorded in KBr on a Jasco FT-IR 5300 (Jasco Inc., Mary’s Court Easton, MD, USA), and the superior absorption was listed in cm−1. 1H-NMR/13C-NMR spectra were taken on an NMR Agilent system consol DD2 spectrometer (Agilent Technologies Inc., Santa Clara, CA, USA) (1H-NMR 500 MHz; 13C-NMR 125 MHz). MS spectra were measured with high-resolution mass spectrometry (Waters Corp., Milford, MA, USA), detector ESI TOF, solvent aceton +0.1% formic acid in acetonitrile-water (1:1). Thin layer chromatography was carried out on aluminium plates coated with silica gel F254 (E Merck, Darmstadt, Germany) using a UV lamp at 254 nm for spot detection.
3.1. 1-(4-Hexylbenzoyl)-3-Methylthiourea
Mixed the N-methylthiourea 2 (4.5 g, 0.05 mol) with triethyl amine (3 mL) in tetrahydrofuran (20 mL) and then 4-hexylbenzoyl chloride 1 (5.62 g, 0.025 mol) in tetrahydrofuran (20 mL) was added slowly at room temperature. The mixture was refluxed for five hours at 75 °C. The mixture was concentrated by evaporating the THF, and saturated sodium bicarbonate (3 mL) was added to the product, and it was washed twice with distilled water (50 mL). The product was recrystallized with hot ethanol to give the 1-(4-hexylbenzoyl)-3-methylthiourea 3 crystals (Figure 1). Yield of the product was 3.2 g (46%), white crystals, m.p. 89–90 °C. 1H-NMR (δ, ppm, CDCl3, 500 MHz): 0.841–0.881 (3H, t, J = 19.5, CH3 aliphatic); 1.255 (3H, s, N-CH3); 1.564–1.642 (8H, m, 4(CH2)); 2.645–2.677 (2H, t, J = 16, CH2 aliphatic); 3.381 (1H, s, O=C-NH-C=S); 7.761–7.320 (1H, m, S=C-NH-); 7.445–7.461 (2H, d, J = 8, H-aromatic); 7.461–7.756 (2H, d, J = 7.5, H-aromatic). 13C-NMR (δ, ppm, CHCl3-DD2, 125 MHz): 14.19; 22.68; 29.03; 31.21; 31.76; 36.04 (C, Hexyl); 42.34 (1C, -CH3); 127.54 (2C, aromatic); 128.85 (2C, aromatic); 132.74 (1C, aromatic), 147.57 (1C, aromatic); 174.84 (1C, -C=O), 186.73 (1C, -C=S). IR (KBr), υmaks (cm−1): 3334 (N-H); 1644 (C=O amide); 1563 (C=C aromatic); 1171.6 (-C=S). HRMS m/z ESI [M + H]+: experiment 279.1530 and calculated 279.1526.
3.2. Cytotoxic Activity
In vitro anticancer activity against HeLa, MCF-7, T47D and WiDr cell lines were assayed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method, and the results were expressed as IC50, the concentration of the compounds inducing a 50% inhibition of cell growth of treated cells, compared to control cells. Hydroxyurea (HU) was used as the reference drug. Cancer cell lines were seeded at a density of 1 × 104 cells/well in 96-well microplates. After 24 h, exponentially growing cells were exposed to the test compounds in DMSO, at final concentrations ranging from 15.625–250 μg/mL. After 48 h of compound exposure in a 5% CO2 incubator at 37 °C, cell survival was determined by the addition of MTT solution (100 μL of 0.5 mg/mL MTT in PBS). Once formazan was formed, 100 μL of 10% SDS in 0.1 N HCl was added, and the plates were incubated in the dark at 37 °C overnight. The absorbance was observed at 595 nm on an ELISA-reader, and the survival ratio of living cells to untreated cells was expressed as a percentage. Each experiment was performed at least three times [23]. The IC50 value of the test compound was shown in Table 1.
4. Conclusions
In summary, we have successfully synthesized the 1-(4-hexylbenzoyl)-3-methylthiourea compound through an acylation reaction between 4-hexylbenzoyl chloride with N-methylthiourea. Overall, the compound was more active on four cancer cell lines (HeLa, MCF-7, T47D and WiDr), when it was compared with hydroxyurea. It showed the best anticancer activity on the T47D cell line, so it can be used as a promising anticancer candidate, although it must be tested further preclinical.
Supplementary Materials
The following are available online at http://www.mdpi.com/1422-8599/2018/3/M1005/s1, Figure S1: IR Spectrum of compound 3, Figure S2: 1H-NMR Spectrum of compound 3, Figure S3: 13C-NMR Spectrum of compound 3, Figure S4: Mass Spectrum of compound 3.
Author Contributions
R.R. did the synthesis and prepared the manuscript. R.M. finished the elucidation of the compound. T.N. and T.L. helped conduct the in vitro test. S.S. edited and revised the manuscript.
Funding
This research was funded by a Doctoral Research Grant from the Ministry of Research, Technology and Higher Education Indonesia in 2015.
Acknowledgments
The authors thank STIKes Bakti Tunas Husada Tasikmalaya, Faculty of Pharmacy of Airlangga University, ITB Bandung, and Faculty of Medicine of Gadjah Mada University for the provision of the facilities to complete our research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lan, J.; Huang, L.; Lou, H.; Chen, C.; Liu, T. Design, and synthesis of novel C14-urea-tetrandrine derivatives with potent anti-cancer activity. Eur. J. Med. Chem. 2018, 143, 1968–1980. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Zhong, Y.; Mai, X.; Liao, J.; Liu, C.; Feng, H. Synthesis and Anticancer Evaluation of Benzyloxyurea Derivatives. Chem. Pharm. Bull. 2014, 62, 898–905. [Google Scholar] [CrossRef] [PubMed]
- North, E.J.; Scherman, M.S.; Bruhn, D.F.; Scarborough, J.S.; Maddox, M.M.; Jones, V. Design, synthesis and anti-tuberculosis activity of 1-adamantyl-3-heteroaryl ureas with improved in vitro pharmacokinetic properties. Bioorg. Med. Chem. 2013, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; North, E.J.; Hurdle, J.G.; Morisseau, C.; Scarborough, J.S.; Sun, D.; Korduláková, J.; Scherman, M.S.; Jones, V.; Grzegorzewicz, A.; et al. The Structure Activity Relationship of Urea Derivatives as Anti-Tuberculosis Agents. Bioorg. Med. Chem. 2011, 19, 5585–5595. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.D.; Skinner-Adams, T.S.; Andrews, K.T.; Coster, M.J.; Edstein, M.D.; Mackenzie, D.; Charman, S.A.; Koltun, M.; Blundell, S.; Campbell, A.; et al. Biomolecular Chemistry Synthesis and antimalarial evaluation of amide and natural product scaffold. Org. Biomol. Chem. 2015, 13, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, J.N.; León, C.; Rodrigues, J.; Gamboa de Domínguez, N.; Gut, J.; Rosenthal, P.J. Synthesis and Evaluation of New Antimalarial Phenylurenyl Chalcone Derivatives. J. Med. Chem. 2005, 48, 3654–3658. [Google Scholar] [CrossRef] [PubMed]
- Sartoril, E.; Camyl, F.; Teulonl, J.M.; Cambordez, F.; Meignen, J.; Hertzz, F. Synthesis and analgesic activities of urea derivatives of a-amino-N-pyridyl benzene propanamide. Eur. J. Med. Chem. 1994, 29, 431–4399. [Google Scholar] [CrossRef]
- Manjula, S.N.; Noolvi, N.M.; Parihar, K.V.; Reddy, S.A.M.; Ramani, V.; Gadad, A.K. European Journal of Medicinal Chemistry Synthesis and antitumor activity of optically active thiourea and their 2-aminobenzothiazole derivatives: A novel class of anticancer agents. Eur. J. Med. Chem. 2009, 44, 2923–2929. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hu, Y.; Yang, Y.S.; Zhang, F.; Zhang, Y.B.; Wang, X.L.; Tang, J.F.; Zhong, W.Q.; Zhu, H.L. Design, modification and 3D QSAR studies of novel naphthalin-containing pyrazoline derivatives with/without thiourea skeleton as anticancer agents. Bioorg. Med. Chem. 2013, 21, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Chen, J.; He, Z.; Sun, W.; Xu, W. Design, synthesis and biological activities of thiourea containing sorafenib analogs as antitumor agents. Bioorg. Med. Chem. 2012, 20, 2923–2929. [Google Scholar] [CrossRef] [PubMed]
- Karakuş, S.; Küçükgüzel, Ş.G.; Küçükgüzel, İ.; De Clercq, E.; Pannecouque, C.; Andrei, G.; Snoeck, R.; Sahin, F.; Bayrak, Ö.F. Synthesis, antiviral and anticancer activity of some novel thioureas derived from N-(4-nitro-2-phenoxyphenyl)-methanesulfonamide. Eur. J. Med. Chem. 2009, 44, 3591–3595. [Google Scholar] [CrossRef] [PubMed]
- Pingaew, R.; Prachayasittikul, V.; Anuwongcharoen, N.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis and molecular docking of N, N′-disubstituted thiourea derivatives as novel aromatase inhibitors. Bioorg. Chem. 2018, 79, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Sett, P.P.; Ranken, R.; Robinson, D.E.; Osgood, S.A.; Risen, L.M.; Rodgers, E.L.; Migawa, M.T.; Jefferson, E.A.; Swayze, E.E. Aryl urea analogs with broad-spectrum antibacterial activity. Bioorg. Med. Chem. Lett. 2004, 14, 5569–5572. [Google Scholar] [CrossRef] [PubMed]
- Upadhayaya, R.S.; Kulkarni, G.M.; Vasireddy, N.R.; Vandavasi, J.K.; Dixit, S.S.; Sharma, V.; Chattapadhayaya, J. Design, synthesis and biological evaluation of novel triazole, urea and thiourea derivatives of quinoline against Mycobacterium tuberculosis. Bioorg. Med. Chem. 2009, 17, 4681–4692. [Google Scholar] [CrossRef] [PubMed]
- Vega-Pérez, J.M.; Periñán, I.; Argandoña, M.; Vega-Holm, M.; Palo-Nieto, C.; Burgos-Morón, E.; López-Lázaro, M.; Vargas, C.; Nieto, J.J.; Iglesias-Guerra, F. Isoprenyl-thiourea and urea derivatives as new farnesyl diphosphate analogs: Synthesis and in vitro antimicrobial and cytotoxic activities. Eur. J. Med. Chem. 2012, 58, 591–612. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Singh, N.; Saleem, K. Synthesis, characterization and in vitro antibacterial activity of thiourea and urea derivatives of stereoids. Eur. J. Med. Chem. 2008, 43, 2272–2277. [Google Scholar] [CrossRef] [PubMed]
- Binzet, G.; Gumus, I.; Dogen, A.; Flörke, U.; Kulcu, N.; Arslan, H. Nickel(II) and Copper(II) Complexes of N,N-Dialkyl-N′-3-Chlorobenzoylthiourea: Synthesis, Characterization, Crystal Structures, Hirshfeld Surfaces and Antimicrobial Activity. J. Mol. Struct. 2018, 1161, 519–529. [Google Scholar] [CrossRef]
- Bielenica, A.; Drzewiecka-Antonik, A.; Rejmak, P.; Stefańska, J.; Koliński, M.; Kmiecik, S.; Lesyng, B.; Włodarczyk, M.; Pietrzyk, P.; Struga, M. Synthesis, Structural and Antimicrobial Studies of Type II Topoisomerase-Targeted Copper(II) Complexes of 1,3-Disubstituted Thiourea Ligands. J. Inorg. Biochem. 2018, 182, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Burmistrov, V.; Morisseau, C.; Pitushkin, D.; Karlov, D.; Fayzullin, R.R.; Butov, G.M.; Hammock, B.D. Adamantyl Thioureas as Soluble Epoxide Hydrolase Inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 2302–2313. [Google Scholar] [CrossRef] [PubMed]
- Burgeson, J.R.; Moore, A.L.; Boutilier, J.K.; Cerruti, N.R.; Gharaibeh, D.N.; Lovejoy, C.E.; Amberg, S.M.; Hruby, D.E.; Tyavanagimatt, S.R.; Allen, R.D.; et al. Analysis of a series of acylthiourea derivatives possessing broad-spectrum antiviral. Bioorg. Med. Chem Lett. 2012, 22, 4263–4272. [Google Scholar] [CrossRef] [PubMed]
- Ruswanto; Miftah, A.M.; Tjahjono, D.H.; Siswandono. Synthesis and in vitro Cytotoxicity of 1-Benzoyl-3-methyl thiourea Derivatives. Procedia Chem. 2015, 17, 157–161. [Google Scholar]
- Gumus, I.; Solmaz, U.; Binzet, G.; Keskin, E.; Arslan, B.; Arslan, H. Hirshfeld Surface Analyses and Crystal Structures of Supramolecular Self-Assembly Thiourea Derivatives Directed by Non-Covalent Interactions. J. Mol. Struct. 2018, 1157, 78–88. [Google Scholar] [CrossRef]
- Ansari, M.F.; Idrees, D.; Hassan, M.I.; Ahmad, K.; Avecilla, F.; Azam, A. Design, synthesis and biological evaluation of novel pyridine-thiazolidinone derivatives as anticancer agents: Targeting human carbonic anhydrase IX. Eur. J. Med. Chem. 2018, 144, 544–556. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).