Abstract
The nitramino derivatives of furoxans are of specific interest as precursors for the preparation of high energy salts with nitrogen-rich cations. In this communication, the 3,3′-(diazene-1,2-diyl)bis[4-(nitroamino)-1,2,5-oxadiazole 2-oxide] was prepared via nitration of available 4,4′-diamino-3,3′-diazenofuroxan; the best yield of the target compound was achieved under the action of nitrating system HNO3/(CF3CO)2O in molar ratio 15:3 in CCl4 at −5 °C. The structure of 3,3′-(diazene-1,2-diyl)bis[4-(nitroamino)-1,2,5-oxadiazole 2-oxide] was confirmed by means of 1H, 13C,14N-NMR, IR spectroscopy and high resolution mass spectra (HRMS).
1. Introduction
The furoxan ring was found to be a useful subunit for the design of new high density, high energy materials (HEDMs) composed exclusively of carbon, hydrogen, nitrogen, and oxygen atoms. The high combined O + N content (69.7%), high density, and positive heat of formation of furoxan make it particularly interesting as an integral component in the construction of new HEDMs. The introduction of furoxan ring is thought to contribute to the detonation performance due to the high enthalpy of formation of the furoxan ring (ΔHfo = 197.8 kJ·mol–1) and the presence of two active oxygen atoms in the molecule [1,2,3,4,5]. Among them, the nitrofuroxans are especially effective [6,7,8]. However, the absence of an acidic proton in the furoxan ring makes it impossible to serve as Brønsted acid or to pair with a Lewis base. Thus, it could not act as a cation or an anion in energetic salts, while, when a furoxan ring replaces a nitro group, the density and detonation velocity of a compound can be increased by about 0.06–0.08 g·cm−3 and 300 m·s−1, respectively.
The nitramino group is an important explosophore that is present in many energetic compounds. The replacement of nitro group with nitramino one in high energy molecules, including furoxan derivatives, enhances the density and heat of formation as well as the detonation properties [9]. Herein, we report the synthesis of 3,3′-(diazene-1,2-diyl)bis[4-(nitroamino)-1,2,5-oxadiazole 2-oxide] 1. This compound may be of interest as a precursor for the preparation of various high energy and thermally stable salts with nitrogen-rich cations for applications in future energetic materials.
2. Results and Discussion
Our research team has a great experience in the synthesis and reactivity of furoxans [10,11,12,13,14]. Therefore, to prepare the target compound 1, we have used the nitration of the previously synthesized [6] 4,4′-diamino-3,3′-diazenofuroxan 2. For this aim, both fuming nitric acid and its mixtures with acetic or trifluoroacetic anhydrides were utilized. The reagents ratio, temperature and additives of organic solvents were varied. A big excess of HNO3 (molar ratio 2:HNO3 = 1:145) at 0 °C did not provide target compound 1—only a total decomposition of starting material was observed (Table 1, entry 1). The negative result was also obtained at nitration of compound 2 with a big excess of the mixture of HNO3 and (CF3CO)2O (molar ratio 2:HNO3:(CF3CO)2O = 1:72:22) at −10 °C (entry 2). A small yield of the target dinitramine 1 was obtained at a decrease of reagents molar ratio (2: HNO3: Ac2O = 1:15:3) and at addition of CCl4 at 0 °C followed by the temperature increase to 20 °C (entry 3). The yield of compound 1 increased at molar ratio 2:HNO3:(CF3CO)2O = 1:15:2.2 in CCl4 at 0 °C (entry 4). The best result was achieved at reagents molar ratio 2:HNO3:(CF3CO)2O = 1:15:3 at −5 °C in CCl4 for 0.5 h (entry 5, Scheme 1).

Table 1.
Optimization of the reaction conditions.
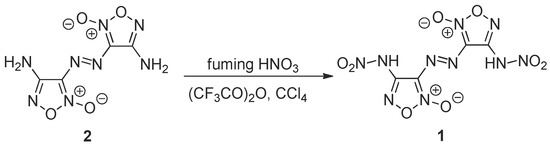
Scheme 1.
Synthesis of 3,3′-(diazene-1,2-diyl)bis[4-(nitroamino)-1,2,5-oxadiazole 2-oxide] 1.
The structure of 3,3′-(diazene-1,2-diyl)bis[4-(nitroamino)-1,2,5-oxadiazole 2-oxide] 1 was strictly confirmed by means of 1H, 13C, 14N-NMR, IR spectroscopy and high resolution mass spectra (HRMS).
3. Experimental Section
3.1. General Information
All reactions were carried out in well-cleaned oven-dried glassware with magnetic stirring. 1H NMR spectrum was recorded on a Bruker AM-300 (300.13 MHz) spectrometer (Bruker, Billerica, MA, USA). 13C-NMR spectrum was recorded on a Bruker AV-600 (150.9 MHz) spectrometer (Bruker) and referenced to residual solvent peak. 14N-NMR spectrum was measured on a Bruker AM-300 (21.69 MHz) spectrometer (Bruker) using MeNO2 (δ14N = 0.0 ppm) as an external standard. The chemical shifts are reported in ppm (δ). The IR spectrum was recorded on a Bruker “Alpha” spectrometer (Bruker) in the range 400–4000 cm−1 (resolution 2 cm−1) as a pellet with KBr. The melting point was determined on Stuart SMP20 apparatus (Stuart, Staffordshire, United Kingdom) and is uncorrected. High resolution mass spectrum was recorded on a Bruker microTOF spectrometer (Bruker) with electrospray ionization (ESI). Measurement was performed in a positive (+MS) ion mode (interface capillary voltage: 4500 V) with scan range m/z: 50–3000. The initial 4,4′-diamino-3,3′-diazenofuroxan 2 was prepared by oxidative condensation of 4-amino-3-azidocarbonylfuroxan under the action of KMnO4 in the presence of hydrochloric acid followed by Curtius rearrangement of azidocarbonyl groups in the formed 4,4′diazeno-3,3′-bis(azidocarbonyl)furoxan at 80 °C and simultaneous isomerization of both furoxan rings [6].
3.2. Synthesis of 3,3′-(Diazene-1,2-diyl)bis[4-(nitroamino)-1,2,5-oxadiazole 2-oxide] 1
Trifluoroacetic anhydride (0.67 mL, 4.8 mmol) was added dropwise to a magnetically stirred solution of fuming HNO3 (1.0 mL, 23.8 mmol) in CCl4 (10 mL) at −5 °C. Then, 3,3′-(diazene-1,2-diyl)bis(4-aminofuroxan) 2 (0.37 g, 1.6 mmol) was added portionwise at −5 °C. The reaction mixture was stirred for 30 min, the resulted solid was filtered off, washed with cold CF3COOH (2 × 4 mL) and dried in a vacuum desiccator over P2O5 and KOH for 24 h. Yield 0.34 g (80%), yellow crystals, m.p. 74–75 °C. IR spectrum, ν, cm−1: 3305 (NH), 1587 (NO2 asym), 1498 (furoxan), 1401 (N=N), 1320, (NO2 sym), 1295 (furoxan), 1157 (furoxan), 1053, 985, 894, 838, 770, 713, 647; 1H-NMR (300 MHz, acetic acid-d4): δ 11.34 (br s, 2H, NH); 13C-NMR (150.9 MHz, acetic acid-d4): δ 111.0 (C-3 furoxan), 158.2 (C-4 furoxan); 14N-NMR (21.7 MHz, acetic acid-d4): δ −38.4 (s, NO2), −25.0 (br. s. furoxan); HRMS (ESI) Calcd for: C4H3N10O8: 319.0130; Found: 319.0138 [M + H]+.
Supplementary Materials
The following are available online, Figure S1: IR spectrum of compound 1, Figure S2: 1H-NMR spectrum of compound 1 in acetic acid-d4, Figure S3: 13C-NMR spectrum of compound 1 in acetic acid-d4, Figure S4: 14N-NMR spectrum of compound 1 in acetic acid-d4, Figure S5: HRMS of compound 1.
Author Contributions
A.L.: experimental synthetic work, IR and NMR interpretation; I.O.: experimental synthetic work, literature research, IR and NMR interpretation; L.F.: literature research, synthesis planning, proofreading of manuscript; N.M.: synthesis planning, literature research, writing of manuscript.
Funding
This research was funded by the Russian Science Foundation grant number 14-50-00126. The APC was funded by N. D. Zelinsky Institute of Organic Chemistry RAS.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, B.; Yang, H.; Lin, Q.; Wang, Z.; Lu, C.; Cheng, G. New thermally stable energetic materials: Synthesis and characterization of guanylhydrazone substituted furoxan energetic derivatives. New J. Chem. 2015, 39, 179–186. [Google Scholar] [CrossRef]
- Klapoetke, T.M.; Witkowski, T.G. Nitrogen-Rich Energetic 1,2,5-Oxadiazole-Tetrazole—Based Energetic Materials. Propellants Explos. Pyrotech. 2015, 40, 366–373. [Google Scholar] [CrossRef]
- Liang, L.; Wang, K.; Bian, C.; Ling, L.; Zhou, Z. 4-Nitro-3-(5-tetrazole)furoxan and Its Salts: Synthesis, Characterization, and Energetic Properties. Chem. Eur. J. 2013, 19, 14902–14910. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Klapoetke, T.M.; Stierstorfer, J. Synthesis and Characterization of Diaminobisfuroxane. Eur. J. Inorg. Chem. 2014, 2014, 5808–5811. [Google Scholar] [CrossRef]
- He, C.; Shreeve, J.M. Potassium 4,5-Bis(dinitromethyl)furoxanate: A Green Primary Explosive with a Positive Oxygen Balance. Angew. Chem. Int. Ed. 2016, 55, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikov, I.V.; Makhova, N.N.; Khmel’nitskii, L.I.; Kuz’min, V.S.; Akimova, L.N.; Pepekin, V.I. Dinitrodiazenofuroxan as a new energetic explosive. Dokl. Chem. 1998, 359, 67–70. [Google Scholar]
- Stepanov, A.I.; Dashko, D.V.; Astrat’ev, A.A. 3,4-Bis(4′-nitrofurazan-3′-yl)furoxan: A Melt Cast Powerful Explosive and a Valuable Building Block in 1,2,5-Oxadiazole Chemistry. Cent. Eur. J. Energ. Mater. 2012, 9, 329–342. [Google Scholar]
- He, C.; Gao, H.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Boosting energetic performance by trimerizing furoxan. J. Mater. Chem. A 2018, 6, 9391–9396. [Google Scholar] [CrossRef]
- He, C.; Tang, Y.; Mitchell, L.A.; Parrish, D.A.; Shreeve, J.M. N-Oxides light up energetic performances: Synthesis and characterization of dinitraminobisfuroxans and their salts. J. Mater. Chem. A 2016, 4, 8969–8973. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Epishina, M.A.; Kulikov, A.S.; Ovchinnikov, I.V.; Ananyev, I.V.; Makhova, N.N. An efficient access to (1H-tetrazol-5-yl)furoxan ammonium salts via a two-step dehydration/[3+2]-cycloaddition strategy. Tetrahedron 2015, 71, 6764–6775. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Makhova, N.N. Advances in the synthesis of non-annelated polynuclear heterocyclic systems comprising the 1,2,5-oxadiazole ring. Russ. Chem. Rev. 2016, 85, 1097–1145. [Google Scholar] [CrossRef]
- Zlotin, S.G.; Churakov, A.M.; Dalinger, I.L.; Luk’yanov, O.A.; Makhova, N.N.; Sukhorukov, A.Y.; Tartakovsky, V.A. Recent advances in synthesis of organic nitrogen–oxygen systems for medicine and material science. Mendeleev Commun. 2017, 27, 535–546. [Google Scholar] [CrossRef]
- Kuchurov, I.V.; Zharkov, M.N.; Fershtat, L.L.; Makhova, N.N.; Zlotin, S.G. Prospective Symbiosis of Green Chemistry and Energetic Materials. ChemSusChem 2017, 10, 3914–3946. [Google Scholar] [CrossRef] [PubMed]
- Fershtat, L.L.; Ovchinnikov, I.V.; Epishina, M.A.; Romanova, A.A.; Lempert, D.B.; Muravyev, N.V.; Makhova, N.N. Assembly of Nitrofurazan and Nitrofuroxan Frameworks for High-Performance Energetic Materials. ChemPlusChem 2017, 82, 1315–1319. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).