Abstract
Novel synthesis of a 2-amino-3-cyano-2,6-diphenyl pyridine by a one-pot multi-component reaction of 1,3-diphenylpropane-1,3-dione, malononitrile and phenethylamine in the presence of N-hydroxybenzamide and zinc chloride has been reported. The structure of the synthesized compound was assigned on the basis of its elemental analysis, 1H-NMR, 13C-NMR, IR and mass spectral data. X-ray structure analysis confirmed unambiguously the proposed structure. The photophysical properties (λAbs., λFlu.) in CH3OH, CH3CN, CH2Cl2 and the emission spectrum of the new compound in solution and in the solid state are reported.
The optoelectronic devices such as optical fibers, switches, tunable lasers and amplifiers, modulators with various applications need compounds emitting in the blue spectral region [1]. So developing a procedure for the synthesis of thermally stable, highly fluorescent materials can be urgently interesting for technology upgrading. There are some reports on using the fluorescent compounds in biochemical and medical research [2]. 2-Aminopyridine derivatives are of great importance due to their biological activities such as cardioprotective [3], antibacterial [4], antioxidant [5], anti-inflammatory [6] and anti-HIV [7]. Sulfapyridine, an example which contains 2-aminopyridine moiety, is an old marked antibacterial drug. Therefore designing new procedures to synthesize 2-aminopyridine derivatives has attracted a lot of interest. Literature shows some reports on the synthesis of 2-amino-3-cyano-4,6-dialkylpyridines but there are few reports on diaryl ones [8,9,10,11]. Recently we have reported the synthesis of 2-amino-3-cyano-4,6-dialkyl pyridines by the one-pot multi-component reaction of 2,4-pentanedione, malononitrile and primary or secondary amines in the presence of N-hydroxy benzamide or p-toluenesulfonic acid as the acidic catalyst [12]. In continuation of our research on the synthesis of fluorescent compounds [12,13,14], we herein report the synthesis of 3-cyano-4,6-diphenyl-2-(phenethylamino) pyridine using N-hydroxybenzamide and ZnCl2 as an organic acid and Lewis acid catalysts respectively (Scheme 1).
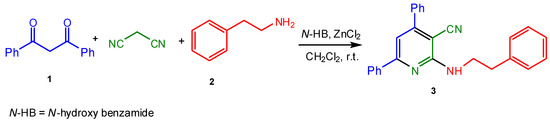
Scheme 1.
Synthesis of 3-cyano-4,6-diphenyl-2-(phenethylamino)pyridine 3.
Structure 3 was assigned on the basis of its elemental analysis, 1H-NMR, 13C-NMR, IR and mass spectral data. The light yellow crystals of 3 were obtained by crystallization from ethyl acetate/n-hexane: 1/3 and its X-ray structure was determined to confirm unambiguously its structure. [15] (Figure 1).
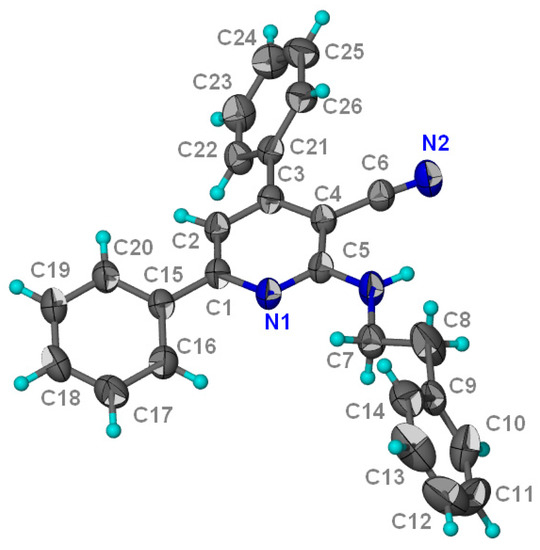
Figure 1.
X-ray crystal structure of 3.
Compound 3 has shown fluorescence activity in the blue region in solution and solid phase. The photophysical data for this compound including λAbs. (nm), λFlu. (nm) have been measured for 0.00002 M solutions in CH3OH, CH3CN and CH2Cl2 (Table 1). The fluorescence emission spectrum of compound 3 solutions in CH2Cl2, CH3CN and CH3OH is shown in Figure 2.

Table 1.
Photophysical data: electronic absorption (Abs.) and fluorescence (Flu.) of 3.
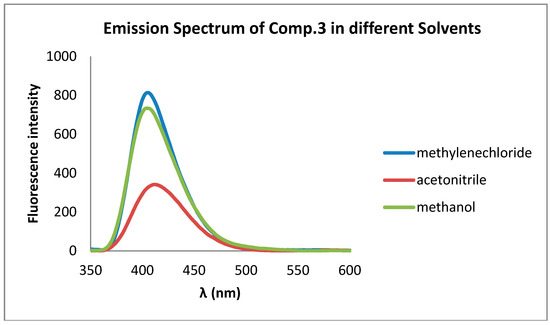
Figure 2.
The fluorescence emission spectrum of compound 3 solutions in CH2Cl2, CH3CN and CH3OH.
Figure 3 shows photographs of compound 3 solutions in CH2Cl2, CH3CN and CH3OH (a): under visible light and (b): under a UV lamp with λ = 366 nm (Philips TL8W/08F8T5/BLC).
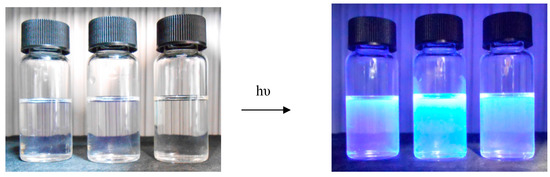
Figure 3.
Photographs of solutions of 3 from left to right in CH2Cl2, CH3OH and CH3CN respectively.
The emission spectrum of compound 3 has been determined. The sample is prepared as the following: a solution of compound 3 (0.5 mL) in CHCl3 (10 −8 mol L−1) was first coated on a quartz glass sheet and dried at room temperature, then placed in a 1 cm length quartz cell. The excitation and emission slits were adjusted on 3 nm. The fluorescence emission intensity of compound 3 after excitation in 300 nm is shown in Figure 4. The photo in the top right of Figure 4 has been taken under a UV lamp with λ = 366 nm (Philips TL8W/08F8T5/BLC), only to show the fluorescence property of compound 3 in solid state It seems the title compound can achieve a good chance among the optoelectronic devices.
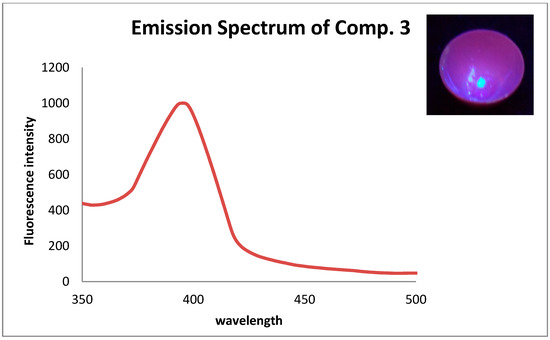
Figure 4.
The fluorescence emission spectrum of compound 3 after excitation in 300 nm. The photo in the top right has been taken under a UV lamp with λ = 366 nm (Philips TL8W/08F8T5/BLC).
Experimental
Elemental analysis for C, H and N was performed using a Thermo Finnigan Flash EA1112 instrument. 1H-NMR and 13C-NMR spectra were determined on a Bruker 250 spectrometer. IR spectra were measured on a Bruker EQUINOX 55 spectrophotometer with the ATR method. Mass spectra were recorded on a Finnigan-MAT 8430 spectrometer. Photophysical data measurements were made by a luminescence PERKIN ELMER LS 50B spectrometer.
3-Cyano-(2-phenethylamino)-4,6-diphenyl pyridine (3). To a magnetically stirred solution of 1,3-diphenylpropane-1,3-dione (0.224 g, 1 mmol) and ZnCl2 (10 mol %) in 10 mL CH2Cl2, malononitrile (0.066 g, 1 mmol), phenethyl amine (0.12 mL, 1 mmol) and N-hydroxy benzamide (50 mol %, 0.68 g) were added in a one-pot manner. The solution was stirred at room temperature for 10 h. The reaction progress was monitored by IR. When one of the CN absorptions (2228 cm−1) in IR spectrum of the reaction mixture had disappeared, the solvent was removed under reduced pressure and the product was purified using column chromatography (silica gel, ethyl acetate/n-hexane: 1/5). The crystals of product were obtained (0.27 g, 72% yield) as yellow crystals. Melting point: 148 °C.
Structural Characterization
IR, νmax: 3353, 2211, 1550 cm−1; δH (250 MHz, CDCl3): 3.08 (2H, t, J = 7. 2 Hz, CH2), 3.96 (2H, q, J = 6.7 Hz, CH2-N), 5.51-5.55 (1H, br s, NH), 7.19 (1H, s, CH of pyridine ring), 7.26–7.42, 7.50–7.59, 7.64–7.68, 8.12–8.15 (15H, 4m, Ph protons) ppm; δC (CDCl3): 35.93 (CH2), 43.25 (CH2-N), 109.61 (CN), 117.41 (CH of pyridine), 126.60, 127.39, 128.76, 128.78, 128.94, 129.73, 130.17, 137.34, 138.34, 139.17, 155.08, 159.06 (aromatic carbons) ppm; MS: m/z = 375 (M+), 298, 272, 255, 221, 195, 120, 91, 77, 75, 43; Anal. Calcd for C26H21N3: C, 83.17; H, 5.64; N, 11.19; Found: C, 83.20; H, 5.69; N, 11.23.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Support of this study by the Research Council at the University of Tehran is gratefully acknowledged.
Author Contributions
All the authors equally contributed to the research. ZI and HS did the experiments. KA performed the spectroscopic data. SWN performed the X-ray structure. AZ wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Duarte, F. Solid-state multiple-prism grating dye-laser oscillator. J. Appl. Opt. 1994, 33, 3857–3860. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of fluorescence spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Stoltefuss, J.; Goldmann, S.; Straub, A.; Boshagen, H.; Bechem, M.; Gross, R.; Hebisch, S.; Hutter, J.; Rounding, H.P. Process for Preparing 1,4-Dihydro-2-amino-3-carboxy-5-cyano-pyridine Derivatives. U.S. Patent 5432282 A, 11 July 1995. [Google Scholar]
- Henry, G.D. De novo synthesis of substituted pyridines. Tetrahedron 2004, 60, 6043–6061. [Google Scholar] [CrossRef]
- Queiroz, M.R.P.; Ferreira, I.C.F.R.; Calhelha, R.C.; Estevinho, L.M. Synthesis and antioxidant activity evaluation of new 7-aryl or 7-heteroarylamino-2,3-dimethyl benzo[b]thiophenes obtained by Buchwald–Hartwig C–N cross-coupling. Bioorg. Med. Chem. 2007, 15, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Manna, F.; Chimenti, F.; Bolasco, A.; Bizzarri, B.; Filippelli, W.; Filippelli, A.; Gagliardi, L. Anti-inflammatory, analgesic and antipyretic 4,6-disubstituted 3-cyano-2-aminopyridines. Eur. J. Med. Chem. 1999, 34, 245–254. [Google Scholar] [CrossRef]
- Deng, J.; Sanchez, T.; Al-Mawsawi, L.Q.; Dayam, R.; Yunes, R.A.; Garofalo, A.; Bolger, M.B.; Neamati, N. Discovery of structurally diverse HIV-1 integrase inhibitors based on a chalcone pharmacophore. Bioorg. Med. Chem. 2007, 15, 4985–5002. [Google Scholar] [CrossRef] [PubMed]
- Bomika, Z.; Andaburskaya, M.; Pelcher, Y.É.; Dubur, G.Y. Some nucleophilic substitution reactions of 2-chloro-3-cyanopyridines. Chem. Heterocycl. Compd. 1976, 12, 896–899. [Google Scholar] [CrossRef]
- Kambe, S.; Saito, K.; Sakurai, A.; Midorikawa, H. A simple method for the preparation of 2-amino-4-aryl-3-cyanopyridines by the condensation of malononitrile with aromatic aldehydes and alkyl ketones in the presence of ammonium acetate. Synthesis 1980, 1980, 366–368. [Google Scholar] [CrossRef]
- Tang, J.; Wang, L.; Yao, Y.; Zhang, L.; Wang, W. One-pot synthesis of 2-amino-3-cyanopyridine derivatives catalyzed by ytterbium perfluorooctanoate [Yb(PFO)3]. Tetrahedron Lett. 2011, 52, 509–511. [Google Scholar] [CrossRef]
- Xin, X.; Huang, P.; Xiang, D.; Zhang, R.; Zhao, F.; Zhang, N.; Dong, D. [5C+ 1N] Annulation of 2,4-pentadienenitriles with hydroxylamine: A synthetic route to multi-substituted 2-aminopyridines. Org. Biomol. Chem. 2013, 11, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Zonouzi, A.; Izakian, Z.; Ng, S.W. Novel synthesis of some new fluorescent 2-amino-3-cyanopyridines. Heterocycles 2012, 85, 2713–2721. [Google Scholar] [CrossRef]
- Zonouzi, A.; Mirzazadeh, R.; Peivandi, A.; Dehdari, S. Efficient synthesis of N-substituted 4-arylquinoline derivatives using ZnCl2 or ZrO2. Heterocycles 2012, 85, 1447–1456. [Google Scholar] [CrossRef]
- Zonouzi, A.; Mirzazadeh, R.; Ng, S.W. Trimethyl 4,6-Dicyano-5-hydroxybenzene-1,2,3-tricarboxylate. Molbank 2012, 2012, M775. [Google Scholar] [CrossRef]
- Crystallographic data for the structure of compound 3 reported in this paper have been deposited with the Cambridge Crystallographie Data Center as supplementary publication No. 983903. CCDC 983903 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.com.ac.uk/data_request/cif. (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).