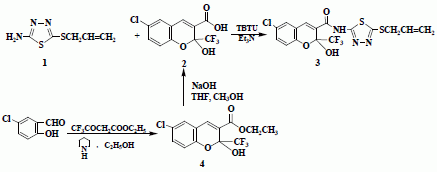
6-Chloro-2-hydroxy-2-trifluoromethyl-2H-chromene-3-carboxylic acid (5-allylsulfanyl-[1,3,4]thiadiazol-2-yl)-amide
Abstract
:1. Introduction
Synthesis
Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Conflicts of Interest
References
- Starks, C.M.; Williams, R.B.; Norman, V.L.; Rice, S.M.; O’Neil-Johnson, M.A.; Lawrence, J.A.; Eldridge, G.R. Antibacterial chromene and chromane stilbenoids from hymenocardia acida. Phytochemistry 2014, 98, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Filipe, A.; Marta, C.; Marian, C.; José, B.; Ellisabet, G.P.; Fernanda, P.; Jordi, M.; Maria, I.L. New chromene scaffolds for adenosine A2A receptors: Synthesis, pharmacology and structure-activity relationship. Eur. J. Med. Chem. 2012, 54, 303–310. [Google Scholar]
- Cheng, J.F.; Akora, I.; Yoshinori, O.; Thomas, A.; Alex, N. Novel chromene derivatives as TNF-α inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 3647–3650. [Google Scholar] [CrossRef] [PubMed]
- Nermien, M.S.; Hany, M.M.; Essam Shawky, A.E.H.K.; Shymaa, S.M.; Ahmed, M.E.A. Synthesis of 4H-chromene, coumarin, 12H-chromeno[2,3-d]pyrimidine derivatives and some of their antimicrobial and cytotoxicity activities. Eur. J. Med. Chem. 2011, 46, 765–772. [Google Scholar]
- Cinzia, C.; Nicolata, D. Synthesis and antirhinovirus activity of new 3-benzyl chromene and chroman derivatives. Bioorg. Med. Chem. 2009, 17, 3720–3727. [Google Scholar]
- Monga, P.K.; Sharma, D.; Dubey, A. Comparative study of microwave and conventional synthesis and pharmacological activity of coumarins: A review. J. Chem. Pharm. Res. 2012, 4, 822–850. [Google Scholar]
- Mohammad, A.; Mehdi, K.; Ali, R.; Saeed, E.; Abdolhossein, Z.; Omidreza, F.; Ramin, M.; Abbas, S. 2H-chromene derivatives bearing thiazolidine-2,4-dione rhodanine or hydantoin moieties as potential anti-cancer agents. Eur. J. Med. Chem. 2013, 59, 15–22. [Google Scholar]
- Yin, S.Q.; Shi, M.; Kong, T.T.; Zhang, C.M.; Han, K.K.; Cao, B.Y.; Zhang, Z.B.; Du, X.L.; Tang, L.Q.; Mao, X.L.; et al. Preparation of S14161 and its analogues and the discoveryof 6-bromo-8- ethoxy-3-nitro-2H-chromene as a more potent antitumor agent in vitro. Bioorg. Med. Lett. 2013, 23, 3314–3319. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.; Doggrell, S.; Hoberg, J.O. Potassium channel activators based on the benzopyran substructure: Synthesis and activity of the C-8 substituent. Bioorg. Med. Chem. 2003, 11, 1663–1668. [Google Scholar] [CrossRef]
- Tatsuya, M.; Kazuya, U.; Osamu, M.; Kazunori, Y.; Noritada, M. Synthetic studies of fluorine-containing compounds for household inseticides. J. Fluor. Chem. 2007, 128, 1174–1181. [Google Scholar]
- Uneyama, K. Organofluorine Chemistry; Blackwell Publishing Ltd.: Oxford, UK, 2006. [Google Scholar]
- Filler, Y.K. Biomedical Aspects of Fluorine Chemistry; Elservier Biomedical Press: New York, NY, USA, 1986. [Google Scholar]
- Hudlicky, M. Chemistry of Organic Fluorine Compounds II; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Pramilla, S.; Pratibha, B.; Manu, S.; Chandr, P.G. Synthesis of formazans from mannich base of 5-(4-chlorophenyl amino)-2-mercapto-1,3,4-thiadiazole as antimicrobial agents. Arab. J. Chem. 2014, 7, 181–187. [Google Scholar]
- Li, Q.; Ren, J.M.; Dong, F.; Feng, Y.; Gu, G.D.; Guo, Z.Y. Synthesis and antifungal activity of thiadiazole-functionalized chitosan derivatives. Carbohydr. Res. 2013, 373, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, S.; Liu, Z.J.; Chen, W.; Fu, J.; Zeng, Q.F.; Zhu, H.L. Synthesis and antimicrobical evalution of a novel class of 1,3,4-thiadiazole: Derivatives bearing 1,2,4-triazolo[1,5-α]pyrimidine moiety. Eur. J. Med. Chem. 2013, 64, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Y.; Wang, C.X.; Fan, S.F.; Zhao, L.J.; Wang, D.; Xu, C.L. Study on the cyclization methods of 3-[1-(phenylhydrazono)ethyl]-chromen-2-ones. Synth. Commun. 2013, 43, 1263–1269. [Google Scholar] [CrossRef]
- Yang, G.Y.; Yang, J.T.; Wang, C.X.; Fan, S.F.; Xie, P.H.; Xu, C.L. Microwave-assisted synthesis of 3-methyl-1-phenylchromeno[4,3-c]pyrazol-4(1H)-ones under solvent-free conditions. Heterocycles 2013, 87, 1327–1336. [Google Scholar] [CrossRef]
- Xu, C.L.; Yang, G.Y.; Wang, C.X.; Fan, S.F.; Xie, L.X.; Gao, Y. An efficient solvent-free synthesis of 2-hydroxy-2-(trifluoromethyl)-2H-chromenes using silica-immobilized L-proline. Molecules 2013, 18, 11964–11977. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, C.; Xu, C.; Yang, G.; Fan, S.; Xin, L.; Guo, T. 6-Chloro-2-hydroxy-2-trifluoromethyl-2H-chromene-3-carboxylic acid (5-allylsulfanyl-[1,3,4]thiadiazol-2-yl)-amide. Molbank 2014, 2014, M830. https://doi.org/10.3390/M830
Wang C, Xu C, Yang G, Fan S, Xin L, Guo T. 6-Chloro-2-hydroxy-2-trifluoromethyl-2H-chromene-3-carboxylic acid (5-allylsulfanyl-[1,3,4]thiadiazol-2-yl)-amide. Molbank. 2014; 2014(3):M830. https://doi.org/10.3390/M830
Chicago/Turabian StyleWang, Caixia, Cuilian Xu, Guoyu Yang, Sufang Fan, Li Xin, and Tingting Guo. 2014. "6-Chloro-2-hydroxy-2-trifluoromethyl-2H-chromene-3-carboxylic acid (5-allylsulfanyl-[1,3,4]thiadiazol-2-yl)-amide" Molbank 2014, no. 3: M830. https://doi.org/10.3390/M830
APA StyleWang, C., Xu, C., Yang, G., Fan, S., Xin, L., & Guo, T. (2014). 6-Chloro-2-hydroxy-2-trifluoromethyl-2H-chromene-3-carboxylic acid (5-allylsulfanyl-[1,3,4]thiadiazol-2-yl)-amide. Molbank, 2014(3), M830. https://doi.org/10.3390/M830