Abstract
The title compound 3-(4-amino-1,2,5-oxadiazol-3-yl)-4-(4-nitro-1,2,5-oxadiazol-3-yl)-1,2,5-oxadiazole (ANFF-1) was synthesized by: (1) by reaction of 3,4-bis(4-nitro-1,2,5-oxadiazol-3-yl)-1,2,5-oxadiazole (BNFF-1) with gaseous ammonia in toluene and (2) by partial oxidation of 3,4-bis(4-amino-1,2,5-oxadiazol-3-yl)-1,2,5-oxadiazole (BAFF-1) with 35% H2O2 in concentrated H2SO4.
Introduction
The relatively high density (1.782 g/mL) together with low melting point (100 °C) makes ANFF-1 (Figure 1a) very attractive as a secondary explosive, oxidizer and melt-castable explosive [1]. It is structurally similar to another heterocyclic energetic compound, 3,4-bis(4-nitro-1,2,5-oxadiazol-3-yl)-1,2,5-oxadiazole-1-oxide (BNFF, Figure 1b) [2,3,4,5,6,7] and may serve as an alternative to BNFF in some weapon applications. In this short note, we report two synthetic methods that were developed for the synthesis of ANFF-1.
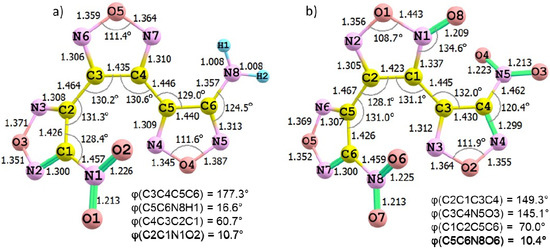
Figure 1.
Geometry structures of (a) ANFF-1 and (b) BNFF molecules were obtained from Density Functional Theory modeling in the B3LYP/6-31+G(2df,p) approximation. The bond distances are shown in Å.
Experimental (Scheme 1)
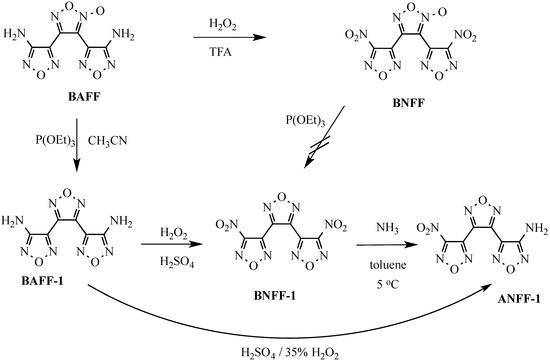
Scheme 1.
Synthesis of ANFF-1.
The first method involved allowing BNFF-1 to react with gaseous ammonia in toluene at 0–5 °C. With stirring, BNFF-1 (5 g) was dissolved in toluene (100 mL) in a 250 mL round-bottomed flask equipped with a stir bar, thermometer and gas bubbler. The mixture was cooled to <5 °C and anhydrous ammonia was bubbled in for 10 min to yield a saturated solution. The mixture was stirred 1 h at <5 °C, filtered to remove insoluble NaNO2 and the solvent was removed to yield a light yellow solid. Purification by flash column chromatography (CH2Cl2, SiO2) yielded 1.4 g of ANFF-1 as a white powder. Recrystallization from CHCl3 yielded white needles; m.p. 100 °C; 1H-NMR (DMSO-d6) δ 6.72 (s, 2H); 13C-NMR (DMSO-d6) δ 160.05, 155.2, 144.25, 140.92, 139.23, 135.70 ppm; 14N-NMR (DMSO-d6) δ 1139 (-NH2) ppm.
The method of choice for the synthesis of ANFF-1 involves the partial oxidation of BAFF-1 with 35% H2O2 in concentrated H2SO4. Into a 300 mL 3-necked round-bottomed flask equipped with a thermometer, stir bar, and addition funnel was placed concentrated (96% sulfuric acid (200 mL) and 3,4-bis(4-amino-1,2,5-oxadiazol-3-yl)-1,2,5-oxadiazole (BAFF-1, 20 g, 84 mmol). The mixture is stirred until a solution is realized. With stirring, the solution is cooled to <10 °C with an ice water bath and 35% hydrogen peroxide (20–30 mL, 8 fold molar excess of H2O2, ~530 mmol) is added dropwise at <20 °C. The mixture turns a blue-green color after an hour BNFF-1 forms as a light blue-green precipitate. The mixture is stirred at 18–23 °C for 3 h. The reaction mixture is filtered through a glass fritted Buchner funnel to remove the insoluble BNFF-1 into 2 liters of ice water, resulting in precipitation of ANFF-1 as a white solid. The precipitate (ANFF-1) is collected by suction filtration, washed with water and allowed to air dry to yield 10.5 g (45%) of a white solid. Recrystallization from CHCl3 (insoluble BAFF-1 is removed by gravity filtration) yields ANFF-1 as white needles; m.p. 100 °C; 1H-NMR (DMSO-d6) δ 6.72 (s, 2H); 13C-NMR (DMSO-d6) δ 160.05, 155.2, 144.25, 140.92, 139.23, 135.70 ppm; 14N-NMR (DMSO-d6) δ 1139 (-NH2) ppm. The resulting material with particles sizes of 0.64 × 0.57 × 0.43 mm3 exhibited friction sensitivity of 0/10@36 kg (BAM Friction) and impact sensitivity measured with drop hammer tests was larger than 177 cm (2.5 kg weight). The two samples had the same particle size and sensitivity since both were recrystallized from CHCl3 prior to performing small-scale safety tests.
Thermal stability of ANFF-1was recently explored and analyzed in great detail [8,9].
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
This research is supported in part by Office of Naval Research (Grant N00014-12-1-0529), DOE, and National Science Foundation (NSF). To perform the most recent calculations, we used NSF XSEDE resources (Grant TG-DMR130077) and Department of Energy NERSC resources (Contract DE-AC02-05CH11231). This work performed in part under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344. MMK is grateful to the Office of the Director of NSF for continuous support under the Independent Research and Development program. Any appearance of findings, conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of NSF.
Author Contributions
All authors equally contributed to preparation of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stepanov, A.I.; Dashko, D.V.; Astrat′ev, A.A. Some Chemical Properties of 3,4-Bis(4-nitrofurazan-3-yl)furoxan. In Proceedings of the 15th International Seminar New Trends in Research of Energetic Materials (NTREM), Pardubice, Czech Republic, April 2012; pp. 301–308.
- Loebbecke, S.; Schuppler, H.; Schweikert, W. Thermal Properties of Different Substituted Energetic Furoxans. In Proceedings of the 33rd International Annual Conference of ICT (Energetic Materials), Karlsruhe, Germany, June 2002; pp. 115/1–115/12.
- Zhao, F.-Q.; Chen, P.; Hu, R.-Z.; Luo, Y.; Zhang, Z.-Z.; Zhou, Y.-S.; Yang, X.-W.; Gao, Y.; Gao, S.-L.; Shi, Q.-Z. Thermochemical Properties and Non-Isothermal Decomposition Reaction Kinetics of 3,4-Dinitrofurazanfuroxan (DNTF). J. Hazard. Mater. 2004, 113, 67–71. [Google Scholar]
- Zheng, W.; Wang, J.-N. Review on 3,4-Bisnitrofurazanfuroxan (DNTF). Chin. J. Energ. Mater. 2006, 14, 463–466. [Google Scholar]
- Pagoria, P.; Hope, M.; Lee, G.; Mitchell, A.; Leonard, P. “Green” Energetic Materials Synthesis at LLNL. In Proceedings of the 15th International Seminar New Trends in Research of Energetic Materials (NTREM), Pardubice, Czech Republic, April 2012; pp. 54–64.
- Zhou, W.-J.; Zhang, G.; Liu, Z.-R. Kinetics of non-isothermal crystallizations of BNFF, TNT and BNFF-TNT eutectic system crystallization in RDX. Hanneng Cailiao 2008, 267–271. [Google Scholar]
- Sinditskii, V.P.; Burzhava, A.V.; Sheremetev, A.B.; Aleksandrova, N.S. Thermal and Combustion Properties of 3,4-Bis(3-nitrofurazan-4-yl)furoxan (DNTF). Propellants Explos. Pyrotech. 2010, 35, 1–6. [Google Scholar] [CrossRef]
- Tsyshesvky, R.; Kuklja, M.M. Decomposition Mechanisms and Kinetics of Novel Energetic Molecules BNFF-1 and ANFF-1: Quantum-Chemical Modeling. Molecules 2013, 18, 8500–8517. [Google Scholar] [CrossRef] [PubMed]
- Kuklja, M.M. Quantum-Chemical Modeling of Energetic Materials: Chemical Reactions Triggered by Defects, Deformations, and Electronic Excitations. In Advances in Quantum Chemistry: Energetic Materials; Sabin, J.R., Brändas, E., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Chapter 3; Volume 68, pp. 71–146. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).