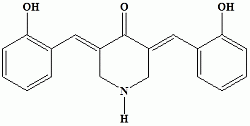
3,5-Bis(2-hydroxybenzylidene)piperidin-4-one
Abstract
:Experimental
Synthesis of 3,5-Bis(2-hydroxybenzylidene)piperidin-4-one (3)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rostom, S.A.F.; Hassan, G.S.; El-Subbagh, H.I. Synthesis and biological evaluation of some polymethoxylated fused pyridine ring system as antitumor agents. Arch. Pharm. (Weinheim, Germany) 2009, 342, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Dandia, A.; Jain, A.K.; Sharma, S. An efficient and highly selective approach for the construction of novel dispiro heterocycles in guanidine-based task-spesific [TMG][Ac] ionic liquid. Tetrahedron Lett. 2012, 53, 5859–5863. [Google Scholar] [CrossRef]
- Kumar, R.R.; Perumal, S.; Senthilkumar, P.; Yogeswari, P.; Stiram, D. An atom efficient, solvent-free, green synthesis and antimycobacterial evaluation of 2-amino-6-methyl-4-aryl-8-[(E)-arylmethylidene]-5,6,7,8-tetrahydro-4H-pyrano[3,2-c]pyridine-3-carbonitriles. Bioorg. Med. Chem. Lett. 2007, 17, 6459–6462. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Nakamura, T.; Iyoki, S.; Fujiwara, A.; Watanabe, Y.; Mohri, K.; Isobe, K.; Ono, K.; Yano, S. Elucidation of anti-allergic activities of curcumin-related compounds with a special reference to their anti-oxidative activities. Biol. Pharm. Bull. 2005, 28, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Handler, N.; Jaeger, W.; Puschacher, H.; Leiser, K.; Erker, T. Synthesis of Novel Curcumin Anologues and Their Evaluation as Selective Cyloxigenase-1(cox-1) Inhibitors. Chem. Pharm. Bull. 2007, 55, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Babasaheb, Y.; Sebastian, T.; Rhonda, J.R.; Schumacher, N.; Diederich, Somer-Edgar, Tiffany, J.; Lesley, L. Synthesis and cytotoxic potential of heterocyclic cyclohexanone analogues of curcumin. Bioorg. Med. Chem. 2010, 18, 6701–6707. [Google Scholar]
- Wichitnithad, W.; Nimmannit, U.; Wacharasindhu, S.; Rojsitthisak, P. Synthesis, Characterization and Biological Evalution of Succinate Pro-drugs of Curcuminoids for Colon Cancer Treatment. Molecules 2011, 16, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Y.; Wang, J.; Weng, B.; Tang, Q.; Chen, X.; Pan, Z.; Liang, G.; Yang, S. Discovery and evaluation of piperid-4-one-containing mono-carbonyl analogs of curcumin as anti-inflammatory agents. Bioorg. Med. Chem. 2013, 21, 3058–3065. [Google Scholar] [CrossRef] [PubMed]

© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Eryanti, Y.; Herlina, T.; Zamri, A.; Halim, S.N.A.; Shiono, Y.; Syah, Y.M.; Awang, K.; Supratman, U. 3,5-Bis(2-hydroxybenzylidene)piperidin-4-one. Molbank 2014, 2014, M825. https://doi.org/10.3390/M825
Eryanti Y, Herlina T, Zamri A, Halim SNA, Shiono Y, Syah YM, Awang K, Supratman U. 3,5-Bis(2-hydroxybenzylidene)piperidin-4-one. Molbank. 2014; 2014(2):M825. https://doi.org/10.3390/M825
Chicago/Turabian StyleEryanti, Yum, Tati Herlina, Adel Zamri, Siti Nadiah Abdul Halim, Yoshihito Shiono, Yana M. Syah, Khalijah Awang, and Unang Supratman. 2014. "3,5-Bis(2-hydroxybenzylidene)piperidin-4-one" Molbank 2014, no. 2: M825. https://doi.org/10.3390/M825
APA StyleEryanti, Y., Herlina, T., Zamri, A., Halim, S. N. A., Shiono, Y., Syah, Y. M., Awang, K., & Supratman, U. (2014). 3,5-Bis(2-hydroxybenzylidene)piperidin-4-one. Molbank, 2014(2), M825. https://doi.org/10.3390/M825