Abstract
A novel symmetrical bisamine derivative (4) was synthesized by condensation reaction of 2,2'-thio-bis[4-methylphenol] (1) and isatoic anhydride in acetonitrile as solvent in the presence of K2CO3 under reflux conditions. The structure of the title compound was established on the basis of elemental analysis, FT-IR, 1H-NMR, 13C-NMR and mass spectral data.
Introduction
Symmetric diaryl sulfides and their sulfoxides have been synthesized by many groups using different conditions [1,2,3]. The Friedel-Crafts method is generally used for the synthesis of these compounds. Treatment of p-cresol with thionyl chloride and anhydrous aluminum chloride in dichloromethane afforded 2,2'-sulfinyl-bis-(4-methylphenol) (1). The reduction of sulfoxide (1) to 2,2'-thio-bis-(4-methylphenol) (2) under different conditions has been reported [4,5,6,7]. Also, dibenzosulfide (2) was synthesized based on a reported procedure in 65% yield [8]. Treatment of the dibenzosulfide 2 with 1-fluoro-2-nitrobenzene and then reduction with NH4Cl/Zn afforded 2,2'-thio-bis-(4-methyl (2-aminophenoxy)phenyl ether] (3) in 90% yield (Figure 1) [9].
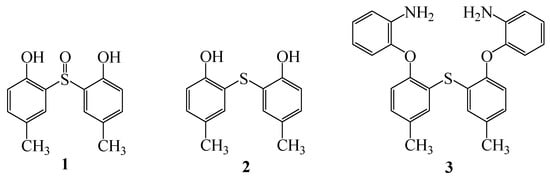
Figure 1.
Examples of sulfoxides and diaryl sulfides.
In addition, the preparation of macrocyclic compounds from dibenzosulfide (2) and diamine (3) have attracted much attention recently, because of their significant metal ion complexing ability as well as being valuable intermediates for the synthesis of aza-crown ethers and related compounds.
In our previous work, we have described the synthesis of new bis-Betti bases [10] and new multibenzo oxygen-sulfur donor macrocycles containing lactams [11] from dibenzosulfide (2) and diamine (3), respectively. In the present study, we report the synthesis of a new bisamine containig a dibenzosulfide moiety. The title compound (4) was synthesized as presented in Scheme 1 by condensation of compound (2) with 2 equivalents of isatoic anhydride in acetonitrile as solvent in the presence of K2CO3 at reflux conditions.
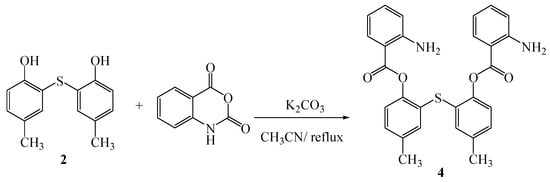
Scheme 1.
Synthetic route to the title compound 4.
Mechanistically, we assume that when dibenzosulfide 2 is treated with 2 equivalents of K2CO3, a thio-bis [4-methyl potassium phenoxide] intermediate is formed which is attacked by 2 equivalents of isatoic anhydride, followed by liberation of 2 equivalents of CO2 to give the title product. The latter can be used for metal ion complexing, synthesis of new aza-crown ethers and related compounds. Identification of product 4 was carried out on the basis of spectroscopic information.
Experimental
All commercially available chemicals and reagents were used without further purification. The melting point was determined with an Electrothermal model 9100 apparatus and is uncorrected. The IR spectrum was recorded on a Shimadzu 4300 spectrophotometer. The 1H and 13C-NMR spectra were recorded in CDCl3 on a Bruker DRX-500 Avance spectrometer. Chemical shifts (δ) are reported in parts per million and are referenced to the NMR solvent. The mass spectrum of the product was obtained with a HP (Agilent technologies) 5937 mass selective detector. Elemental analyses were carried out by a CHN–O–Rapid Heraeus elemental analyzer (Wellesley, MA).
Synthesis of 2,2'-thio-bis[(4-methylphenyl)-2-aminobenzoate (4): A mixture of of 2,2'-thio-bis[4-methylphenol] (1 mmol), isatoic anhydride (2 mmol) and K2CO3 (2 mmol) in acetonitrile (10 mL) was refluxed for 12 h. After completion, the reaction mixture was cooled to room temperature and 10% aqueous KOH (40 mL) was added to the mixture. The white precipitate that formed was filtered, washed with H2O and dried. The product thus obtained was found to be pure upon TLC examination. Yield: 85%; m.p.: 194–196 °C; IR (KBr): 3480 (NH2), 3370 (NH2), 3023, 2913, 1695 (C=O), 1611, 1480, 1240, 1033, 870 cm−1; 1H-NMR (500 MHz, CDCl3): (δ) 2.22 (s, 6H, CH3), 5.65 (s, 4H, NH2), 6.59–7.81 (m, 14H, Ar-H) ppm; 13C-NMR (125 MHz, CDCl3): (δ) 20.69, 109.53, 116.27, 116.46, 123.04, 127.43, 129.36, 131.91, 133.85, 134.65, 136.22, 147.91, 151.11, 166.08 ppm; EI-MS: m/z = 484 (M+), 365, 246, 146, 120, 108, 92; Anal. calcd. for C28H24N2O4S: C, 69.42; H, 4.96; N, 5.78. Found: C, 69.53; H, 4.84; N, 5.81.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
The authors wish to thank the Research Council of the Payame Noor University for financial supports.
References and Notes
- Gazdar, M.; Smiles, S. Aromatic hydroxy-sulphoxides. J. Chem. Soc. Trans. 1910, 97, 2248–2253. [Google Scholar] [CrossRef]
- Gump, W.S.; Vitucci, J.C. 2-Hydroxyphenyl sulfoxides and 2-hydroxyphenyl sulfones. J. Am. Chem. Soc. 1945, 67, 238–240. [Google Scholar] [CrossRef]
- Jung, M.E.; Jachiet, D.; Khan Saeed, I.; Kim, C. New method for the preparation of o-aryloxyphenols: Pummerer-type rearrangement of an o-hydroxyaryl sulfoxide. Tetrahedron Lett. 1995, 36, 361–364. [Google Scholar] [CrossRef]
- Cogan, D.A.; Liu, G.; Kim, K.; Backes, B.J.; Ellman, J.A. Catalytic asymmetric oxidation of tert-butyl disulfide. synthesis of tert-butanesulfinamides, tert-butyl sulfoxides, and tert-butanesulfinimines. J. Am. Chem. Soc. 1998, 120, 8011–8019. [Google Scholar] [CrossRef]
- Schmidt, H.; Bashirpoor, M.; Rehder, D. Structural characterization of possible intermediates in vanadium-catalysed sulfide oxidation. J. Chem. Soc. Dalton Trans. 1996, 3865–3870. [Google Scholar] [CrossRef]
- Jouen, C.; Lasne, M.C.; Pammelet, J.C. Synthesis of α-fluorinated-α,α-difunctionalized sulfides and sulfones. Tetrahedron Lett. 1996, 37, 2413–2416. [Google Scholar] [CrossRef]
- Cubbage, J.W.; Tetzlaff, T.A.; Groundwater, H.; McCulla, R.D.; Nog, M.; Jenks, W.S. Bimolecular photoreduction of aromatic sulfoxides. J. Org. Chem. 2001, 66, 8621–8628. [Google Scholar] [CrossRef] [PubMed]
- Shockravi, A.; Alizadeh, R.; Aghabozorg, H.; Moghimi, A.; Rostami, E.; Bavilli, S. Synthesis and crystal structure determination of 2,2'-sulfinyl-bis(4-methyl phenol) and 2,2'-thio-bis (4-methyl phenol). Phosphorus Sulfur Silicon Relat. Elem. 2003, 178, 2519–2527. [Google Scholar] [CrossRef]
- Shockravi, A.; Chaloosi, M.; Zakeri, M.; Mozaffari, S.; Rostami, E.; Abouzari- Lotf, E. The synthesis and characterization of novel dibenzosulfide diamine and the application in the determination of heavy metals. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 2321–2326. [Google Scholar] [CrossRef]
- Shockravi, A.; Sadeghpour, M.; Olyaei, A. Solvent-and catalyst-free synthesis of new unsymmetrical multidentate thio-bis aminophenol ligands by Mannich condensation. Synth. Commun. 2009, 39, 2347–2359. [Google Scholar] [CrossRef]
- Shockravi, A.; Sadeghpour, M.; Zakeri, M.; Abouzari-Lotf, E.; Olyaei, A. Synthesis of new multibenzo oxygen-sulfur donor macrocycles containing lactams at room temperature. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 808–815. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).