Abstract
A new Schiff base ester, 4-{[(4-chlorophenyl)imino]methyl}-3-hydroxyphenyl 4-(hexadecanoyloxy)benzoate was synthesized and its IR, 1H-NMR, 13C-NMR and MS spectroscopic data are presented.
Benzylideneanilines (Schiff bases) exhibit very interesting thermochromic and photochromic properties. Therefore, they became a topic of numerous recent publications [1,2,3,4,5,6]. Flexible long alkyl chain at the para position of N-benzylideneanilines has also been viewed as one of the important criteria for exhibition of liquid crystal phases [7,8,9,10]. Different alkyl chain length and terminal substituent can significantly influence the anisotropic properties of liquid crystals [7,8]. Thus, we report here another new derivative containing an hexadecanoyloxy chain, 4-{[(4-chlorophenyl)imino]methyl}-3-hydroxyphenyl 4-(hexadecanoyloxy)benzoate.
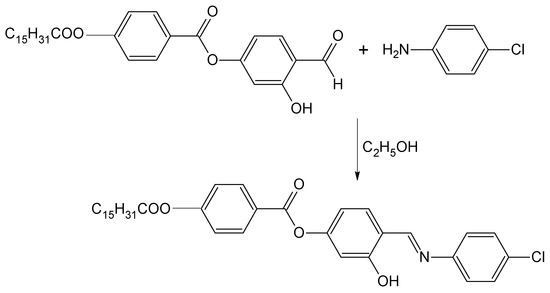
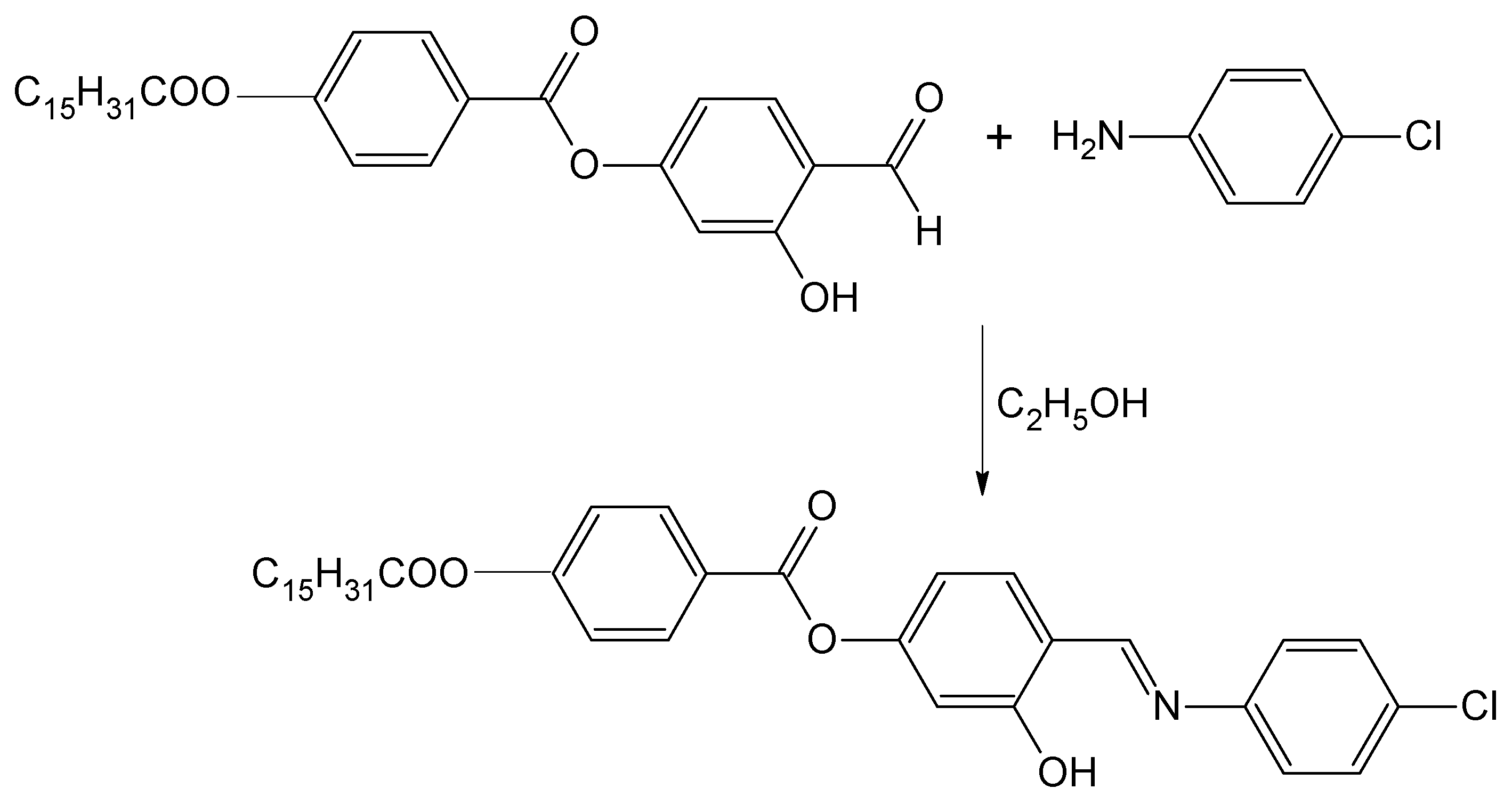
Scheme 1.
Synthesis of 4-{[(4-chlorophenyl)imino]methyl}-3-hydroxyphenyl 4-(hexadecanoyloxy)benzoate.
Scheme 1.
Synthesis of 4-{[(4-chlorophenyl)imino]methyl}-3-hydroxyphenyl 4-(hexadecanoyloxy)benzoate.
Experimental
4-(4-n-Hexadecanoyloxybenzoyloxy)-2-hydroxybenzaldehye was prepared according to method that described in our previous work [11]. In a round-bottom flask, a mixture of the aldehyde (2.48 g, 5.0 mmol), 4-chloroaniline (0.64 g, 5.0 mmol) and absolute ethanol (40 mL) was refluxed with stirring for 3 h. The reaction mixture was filtered and the solvent was removed from the filtrate by evaporation. Recrystallization from absolute ethanol gave the title compound as a yellow solid (1.88 g, 62%).
Melting point: 214–215 °C.
MS (EI): m/z (rel. int. %) = 606 (1) (M+).
IR (KBr): νmax/ cm−1 3400 (broad, O-H), 2951, 2916, 2848 (C-H aliphatic), 1755 (C=O of C15H31COO- fragment), 1743 (C=O of benzoate), 1625 (C=N), 1604 (C=C aromatic), 1282 (C-O).
1H-NMR (400 MHz, CDCl3): δ/ppm 0.92 (t, 3H, J = 6.7 Hz, CH3-), 1.25–1.53 (m, 24H, CH3-(CH2)12-), 1.80 (quint, 2H, J = 7.4 Hz, -CH2-CH2COO-), 2.62 (t, 2H, J = 7.5 Hz, -CH2-COO-), 6.87 (dd, 1H, J = 2.2, 8.4 Hz, Ar-H), 6.94 (d, 1H, J = 2.1 Hz, Ar-H), 7.23–7.29 (m, 4H, Ar-H), 7.40–7.43 (m, 2H, Ar-H), 7.45 (d, 1H, J = 8.4 Hz, Ar-H), 8.25 (d, 2H, J = 8.6 Hz, Ar-H), 8.63 (s, 1H, CH=N), 13.26 (s, 1H, OH).
13C-NMR (100 MHz, CDCl3): δ/ppm 171.9 (C=O of C15H31COO-), 164.3 (C=O of benzoate), 162.5 (C=N), 163.0, 155.7, 155.3, 147.3, 133.7, 133.1, 132.2, 130.0, 127.1, 122.8, 122.3, 117.6, 113.4 and 111.0 for aromatic carbons, 34.8 (-CH2COO-), 25.2 (-CH2CH2COO-), 32.3, 30.1, 30.0, 29.9, 29.8, 29.7, 29.6, 29.5, 23.0 (CH3(CH2)12), 14.4 (CH3(CH2)12).
Elemental analysis: Calculated for C36H44NO5Cl, 71.33%, H, 7.32%, N, 2.31%; Found: C, 71.36%, H, 7.30%, N, 2.32%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
Authors would like to thank Universiti Tunku Abdul Rahman and Universiti Sains Malaysia for the financial supports and research facilities.
References and Notes
- Kosar, B.; Albayrak, C.; Ersanli, C.C.; Odabasoglu, M.; Buyukgungor, O. Molecular structure, spectroscopic investigations, second-order nonlinear optical properties and intramolecular proton transfer of (E)-5-(diethylamino)-2-[(4-propylphenylimino)methyl]phenol: A combined experimental and theoretical study. Spectrochim. Acta A 2012, 93, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hadjoudis, E.; Yannakopoulou, K.; Chatziefthimiou, S.D.; Paulidou, A.; Mavridis, I.M. Supramolecular control of photochromism in a β-cyclodextrin/Schiff base system. J. Photochem. Photobiol. A Chem. 2011, 217, 293–298. [Google Scholar] [CrossRef]
- Naganagowda, G.; Petsom, A. 4-Amino-N-(2-hydroxy-4-pentadecylbenzylidene)-benzenesulfonamide. Molbank 2011, 2011, M739. [Google Scholar] [CrossRef]
- Hadjoudis, E.; Rontoyianni, A.; Ambroziak, K.; Dziembowska, T.; Mavridis, I.M. Photochromism and thermochromism of solid trans-N,N'-bis(salicylidene)-1,2-cyclohexanediamines and trans-N,N'-bis-(2-hydroxynaphylidene)-1,2-cyclohexanediamine. J. Photochem. Photobiol. A Chem. 2004, 162, 521–530. [Google Scholar] [CrossRef]
- Oshima, A.; Momotake, A.; Arai, T. Photochromism, thermochromism, and solvatochromism of naphthalene-based analogues of salicylideneaniline in solution. J. Photochem. Photobiol. A Chem. 2004, 162, 473–479. [Google Scholar] [CrossRef]
- Yeap, G.Y.; Ha, S.T.; Ishizawa, N.; Suda, K.; Boey, P.L.; Mahmood, W.A.K. Synthesis, crystal structure and spectroscopic study of para substituted 2-hydroxy-3-methoxybenzalideneanilines. J. Mol. Struct. 2003, 658, 87–99. [Google Scholar] [CrossRef]
- Yeap, G.Y.; Ha, S.T.; Lim, P.L.; Boey, P.L.; Ito, M.M.; Sanehisa, S.; Youhei, Y. Synthesis, physical and mesomorphic properties of Schiff’s base esters containing ortho-, meta- and para-substituents in benzylidene-4'-alkanoyloxyanilines. Liq. Cryst. 2006, 33, 205–211. [Google Scholar] [CrossRef]
- Godzwon, J.; Sienkowska, M.J.; Galewski, Z. Liquid-crystalline polymorphism of 4-heptyloxybenzylidene-4'- alkyloxyanilines and their phase equilibrium with 4-octyloxyphenyl 4-nitrobenzoate. Thermochim. Acta 2012, 531, 75–82. [Google Scholar] [CrossRef]
- Ha, S.T.; Ong, L.K.; Wong, J.P.W.; Yeap, G.Y.; Lin, H.C.; Ong, S.T.; Koh, T.M. Mesogenic Schiff’s base ether with dimethylamino end group. Phase Transit. 2009, 82, 387–397. [Google Scholar] [CrossRef]
- Ha, S.T.; Ong, L.K.; Ong, S.T.; Yeap, G.Y.; Wong, J.P.W.; Koh, T.M.; Lin, H.C. Synthesis and mesomorphic properties of new Schiff base esters with different alkyl chains. Chin. Chem. Lett. 2009, 20, 767–770. [Google Scholar] [CrossRef]
- Yeap, G.Y.; Ha, S.T.; Boey, P.L.; Mahmood, W.A.K.; Ito, M.M.; Youhei, Y. Synthesis and characterization of some new mesogenic Schiff base esters N-[4-(4-n-hexadecanoyloxybenzoyloxy)benzylidene]-4-substituted anilines. Mol. Cryst. Liq. Cryst. 2006, 452, 73–90. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
