Abstract
One-pot two-step Hantzsch synthesis of 4-(3,5-dibromo-4-hydroxyphenyl)-3- butoxycarbonyl-5-ethoxycarbonyl-2-methyl-6-phenyl-1,4-dihydropyridine under solvent- and catalyst-free conditions promoted with microwave irradiation is presented.
1,4-Dihydropyridines (DHPs) have attracted immense attention for synthetic and medicinal chemists due to their biological and pharmacological properties [1]. 1,4-DHPs, as a class of calcium modulators, are extensively investigated because of their pharmacological activity as drugs in vasodilatation, hepatoprotection, neuromodulatory, cognition, memory enhancing, neuroprotection, anti-atherosclerosis, anti-diabetes, antioxidant, anti-mutagenic, and anti-tumor [2]. The most notable examples are the 1,4-DHP based calcium channel blocker drugs such as nifedipine, felodipine, and nicardipine (Figure 1), which are widely used for the treatment of hypertension and related cardiovascular diseases [3,4].
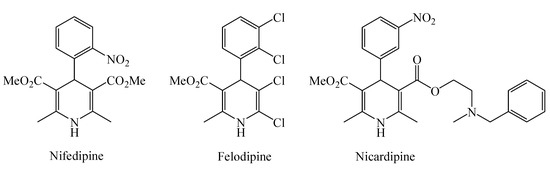
Figure 1.
1,4-DHP drugs for cardiovascular diseases.
In order to model and understand these biological properties and to develop new chemotherapeutic agents based upon the 1,4-DHP motif, considerable effort has been devoted to establish efficient and practical formation methods. The classical synthesis of symmetrical 1,4-DHPs is the three-component one-pot Hantzsch condensation reaction of aldehydes, β-ketoesters, and ammonia or amines [5]. Many improvements and modifications have been developed, including the use of ionic liquids [6], high temperatures at reflux [7] and catalysts such as boronic acids [8], metal triflates [9], molecular iodine [10], TMS iodide [11], Bu4NHSO4 [12], bakers’ yeast [13], ceric ammonium nitrate [14], HCl generated in situ [15], and silica-supported acids [16]. Although most of these processes offer distinct advantages, they suffer from certain drawbacks such as longer reaction times, unsatisfactory yields, high costs, harsh reaction conditions, and the use of a large quantity of volatile organic solvents.
The synthesis of bioactive molecules should preferably be facile, fast, and efficient with minimal workup [17]. Microwave irradiation is an extremely powerful tool for reducing reaction time and increasing desired product yields [18,19]. Unsymmetrical 1,4-DHPs represent effective drug moieties (Felodipine 3, for example) and sometimes possess even better pharmacological activities [20,21,22].
Herein, we reported a one-pot two-step Hantzsch synthesis of 4-(3,5-dibromo-4-hydroxyphenyl)-3-butoxycarbonyl-5-ethoxycarbonyl-2-methyl-6-phenyl-1,4-dihydropyridine under solvent- and catalyst-free conditions promoted with microwave irradiation, as shown in Scheme 1. The title compound has been fully characterized by NMR (1H and 13C), IR, MS, and elemental analysis. This protocol is proven to be facile, efficient, practical, and environmentally benign.
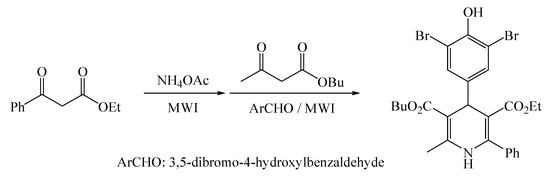
Scheme 1.
Synthesis of the title compound 1.
Experimental
A mixture of ethyl benzoylacetate (0.96 g, 5 mmol) and ammonium acetate (0.77 g, 10 mmol) was heated at 75 °C for 15 min under microwave irradiation (600 W). Then, butyl acetoacetate (0.79 g, 5 mmol) and 3,5-dibromo-4-hydroxybenzaldehyde (1.40 g, 5 mmol) were added, and the mixture was heated at 75 °C for 15 min under microwave irradiation (600 W). After completion of the reaction as monitored by TLC, the crude mixture was purified by flash column chromatography on silica gel (300–400 mesh) eluted with ethyl acetate-petroleum ether (1:7, v/v) to afford the pure product 1 as pale yellow crystals, 2.36 g, 80% yield, mp 184–185 °C.
Structural Characterization
1H NMR (Bruker 300 MHz, DMSO-d6): δH 9.74 (br. s, 1 H, NH), 9.18 (s, 1 H, OH), 7.38 (m, 7 H, Ar-H), 4.82 (s, 1 H, CH), 4.05 (q, 2 H, J = 6.2 Hz, OCH2CH3), 3.70 (q, 2 H, J = 7.1 Hz, OCH2CH2CH2CH3), 2.31 (s, 3 H, CH3), 1.53 (m, 2 H, J = 7.6 Hz, OCH2CH2CH2CH3), 1.27 (m, 2 H, J = 7.5 Hz, OCH2CH2CH2CH3), 0.87 (t, 3 H, J = 7.3 Hz, OCH2CH3), 0.71 (t, 3 H, J = 7.0 Hz, OCH2CH2CH2CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 167.2, 166.6, 147.8, 145.8, 144.6, 142.4, 136.5, 131.6, 129.3, 129.0, 128.4, 128.0, 109.4, 104.1, 103.1, 64.0, 59.8, 39.1, 30.8, 19.6, 19.4, 13.8, 13.6 ppm. IR: υmax 3290, 1679, 1475, 1213, 789, 697 cm−1. MS (AGILENT 5973N MSD, EI): m/z 593.2 (M+). Elemental anal. (Perkin Elmer PE 2400 II HONS): calcd for C26H27Br2NO5 (593.30): C, 52.63; H, 4.59; N, 2.36. Found: C, 52.75; H, 4.53; N, 2.30.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Kappe, C.O. Biologically active dihydropyrimidones of the Biginelli-type — a literature survey. Eur. J. Med. Chem. 2000, 35, 1043–1052. [Google Scholar] [CrossRef]
- Nuhrich, A.; Zemb, V.; Devaux, G.; Vacher, P.; Vacher, A.M.; Dufy, B. Synthesis and activities of a thienyl dihydropyridine series on intracellular calcium in a rat pituitary cell line. Eur. J. Med. Chem. 1996, 31, 547–556. [Google Scholar]
- Lavilla, R. Recent developments in the chemistry of dihydropyridines. J. Chem. Soc. Perkin 1 2002, 9, 1141–1156. [Google Scholar] [CrossRef]
- Alker, D.; Campbell, S.F.; Cross, P.E.; Burges, R.A.; Carter, A.J.; Gardiner, D.G. Synthesis and structure-activity relationships for a series of basic and nonbasic derivatives of 2[(2-aminoethoxy)methyl]-1,4-dihydropyridine calcium antagonists. J. Med. Chem. 1990, 33, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Hantzsch, A. Condensationprodukte aus Aldehydammoniak und Ketoniartigen Verbindungen. Chem. Ber. 1881, 14, 1637–1638. [Google Scholar] [CrossRef]
- Ji, S.J.; Jiang, Z.Q.; Lu, J. Facile Ionic Liquids-Promoted One-Pot Synthesis of Polyhydroquinoline Derivatives under Solvent Free Conditions. Synlett 2004, 5, 831–835. [Google Scholar] [CrossRef]
- Massi, A.; Minghini, E.; Sabbatini, S.; Bertoasi, V. Model Studies toward the Synthesis of Dihydropyrimidinyl and Pyridyl α-Amino Acids via Three-Component Biginelli and Hantzsch Cyclocondensations. J. Org. Chem. 2003, 68, 6172–6183. [Google Scholar]
- Debache, A.; Boulcina, R.; Belfaitah, A.; Rhouati, S.; Carboni, B. One-Pot Synthesis of 1,4-Dihydropyridines via a Phenylboronic Acid Catalyzed Hantzsch Three-Component Reaction. Synlett 2008, 4, 509–512. [Google Scholar] [CrossRef]
- Wang, L.M.; Sheng, J.; Zhang, L.; Han, J.W.; Fan, Z.Y.; Tian, H.; Qian, C.T. Facile Yb(OTf)3 promoted one-pot synthesis of polyhydroquinoline derivatives through Hantzsch reaction. Tetrahedron 2005, 61, 1539–1543. [Google Scholar] [CrossRef]
- Ko, S.; Sastry, M.N.V.; Lin, C.; Yao, C.F. Molecular iodine-catalyzed one-pot synthesis of 4-substituted-1,4-dihydropyridine derivatives via Hantzsch reaction. Tetrahedron Lett. 2005, 46, 5771–5774. [Google Scholar] [CrossRef]
- Sabitha, G.; Reddy, G.S.K.; Reddy, C.S.; Yadav, J.S. A novel TMSI-mediated synthesis of Hantzsch 1,4-dihydropyridines at ambient temperature. Tetrahedron Lett. 2003, 44, 4129–4131. [Google Scholar] [CrossRef]
- Tewari, N.; Dwivedi, N.; Tripathi, R.P. Tetrabutylammonium hydrogen sulfate catalyzed eco-friendly and efficient synthesis of glycosyl 1,4-dihydropyridines. Tetrahedron Lett. 2004, 45, 9011–9014. [Google Scholar] [CrossRef]
- Lee, J.H. Synthesis of Hantsch 1,4-dihydropyridines by fermenting bakers’ yeast. Tetrahedron Lett. 2005, 46, 7329–7330. [Google Scholar] [CrossRef]
- Ko, S.; Yao, C.F. Ceric Ammonium Nitrate (CAN) catalyzes the one-pot synthesis of polyhydroquinoline via the Hantzsch reaction. Tetrahedron 2006, 62, 7293–7299. [Google Scholar] [CrossRef]
- Sharma, G.V.M.; Reddy, K.L.; Lakshmi, P.S.; Krishna, P.R. ‘In situ’ Generated ‘HCl’ - An Efficient Catalyst for Solvent-Free Hantzsch Reaction at Room Temperature: Synthesis of New Dihydropyridine Glycoconjugates. Synthesis 2006, 1, 55–58. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, R.; Paul, S.; Loupy, A. Covalently Anchored Sulfonic Acid on Silica Gel as an Efficient and Reusable Heterogeneous Catalyst for the One-Pot Synthesis of Hantzsch 1,4-Dihydropyridines under Solvent-Free Conditions. Synthesis 2007, 2835–2838. [Google Scholar] [CrossRef]
- Edwards, P.J.; Allart, B.; Andrews, M.J.I.; Clase, J.A.; Menet, C. Expecting drug discovery: Recent advances in fast medicinal chemistry – optimization of hits and leads. Curr. Opin. Drug Discover. Dev. 2006, 9, 425–444. [Google Scholar]
- de la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.A.; Strauss, C.R. Toward Rapid, “Green”, Predictable Microwave– Assisted Synthesis. Acc. Chem. Res. 2005, 38, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Shan, R.; Velazquez, C.; Knaus, E.E. Syntheses, Calcium Channel Agonist− Antagonist Modulation Activities, and Nitric Oxide Release Studies of Nitrooxyalkyl 1,4-Dihydro-2,6-dimethyl-3-nitro-4-(2,1,3-benzoxadiazol-4-yl pyridine-5-carboxylate Racemates, Enantiomers, and Diastereomers. J. Med. Chem. 2004, 47, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.J.; Cao, W.G.; Tong, W.Q. The Knoevenagel condensation reaction of acromatic aldehydes with malononitrile by grinding in the absence of solvents and catalysts. Synth. Commun. 2002, 32, 3475–3479. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, P.; Kapoor, K.K.; Hundal, M.S. An efficient, catalyst- and solvent-free, four-component, and one-pot synthesis of polyhydroquinolines on grinding. Tetrahedron 2008, 64, 536–542. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).