Abstract
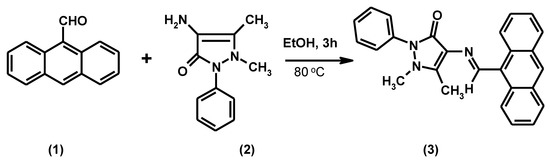
The title compound, 4-[(anthracen-9-ylmethylene)amino]-1,5-dimethyl-2-phenyl-1,2-dihydropyrazol-3-one (3), was synthesized in high yield by reaction of anthracene-9-carbaldehyde and 4-aminoantipyrine in ethanol. The structure of this new compound was confirmed by elemental analysis, IR, 1H NMR, 13C NMR and GC-MS spectral data.
Nitrogen-atom containing heterocyclic compounds are an important subset of the natural products that exhibit biological activities, including antitumor [1], antiamoebic [2], antimicrobial [3] and anti-inflammatory [4] activities. Pyrazol-3-one presents an interesting group of compounds, many of which possess widespread pharmacological properties such as analgesic, antipyretic, and antirheumatic activities [5]. These derivatives are also well known for their pronounced anti-inflammatory properties [6] and are used as potent antidiabetic agents [7] Pyrazol-3-one containing Schiff bases can show even increased biological activity [8]. Since the pyrazol-3-one Schiff base moiety seems to be a possible pharmacophore in various pharmacologically active agents, we decided to synthesize a new pyrazol-3-one containing a Schiff base unit by reaction of anthracene-9-carbaldehyde with 4-aminoantipyrine.
Experimental
A mixture of anthracene-9-carbaldehyde (0.50 g, 0.0024 mol) and 4-aminoantipyrine (0.49 g, 0.0024 mol) in ethanol (15 mL) was heated for 3 h at 80 °C. The reaction was monitored by TLC (chloroform/methanol, 9:1). The solid that separated from the cooled mixture was collected and recrystallized from a methanol/chloroform mixture (8:2) to give the title compound (3) as a yellow solid.
Yield: 87%; m.p. 231–232 °C
GC-MS m/z (rel. int.%): 393 (68) [M+1]+
IR (KBr) vmax cm-1: 3027 (Ar-H, stretch), 2874 (C-H), 1636 (C=O), 1580 (HC=N), 1138 (C-N)
1H NMR (600 MHz, CDCl3) (δ/ppm): 11.06 (s, CHolefinic), 8.98 (d, J = 8.84 Hz, CH), 8.50 (d, J = 7.4 Hz, CH), 8.04 (dd, J = 7.6 Hz, CH), 7.50 (dd, J = 7.2 Hz, CH), 7.48 (s, CH), 7.56–7.51 (m, 5H, CH), 3.23 (s, CH3), 2.19 (s, CH3)
13C NMR (150 MHz, CDCl3) δ: 160.84, 157.70, 152.03, 134.75, 131.54, 130.45, 129.32, 129.01, 128.8, 127.11, 126.60, 125.64, 125.22, 124.62, 119.70, 35.78, 10.36
Anal. calc. for C26H21N3O: C, 79.77, H, 5.41, N, 10.73. Found: C, 79.74, H, 5.38, N, 10.68.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
The authors would like to thank the Chemistry Department, King Abdul Aziz University, Jeddah, Saudi Arabia for providing the research facilities.
References
- Brzozowski, Z.; Czewski, F.S.; Gdaniec, M. Synthesis, structural characterization and antitumor activity of novel 2,4-diamino-1,3,5-triazine derivatives. Eur. J. Med. Chem. 2000, 35, 1053–1064. [Google Scholar] [CrossRef]
- Husain, K.; Abid, M.; Azam, A. Novel Pd(II) complexes of 1-N-substituted 3-phenyl-2-pyrazoline derivatives and evaluation of antiamoebic activity. Eur. J. Med. Chem. 2008, 43, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.; Yusuf, M.; Khan, S.A.; Sahota, P.P.; Pandove, G. Synthesis, studies and invitro-antibacterial activity of N- substituted-5-(furan-2-yl)-phenyl pyrazolines. Arabian J. Chem. 2011, in press. [Google Scholar]
- Amir, M.; Kumar, H.; Khan, S.A. Synthesis and pharmacological evaluation of pyrazoline derivatives as new anti-inflammatory and analgesic agents. Bioorg. Med. Chem. Lett. 2008, 18, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Menozzi, G.; Mosti, L.; Merello, L.; Piana, A.; Armani, U.; Ghia, M.; Angiola, M.; Mattioli, F. 4-Dialkylamino-1-(5-substituted or unsubstituted 1-phenyl-1H-pyrazol-4-yl)butan-1-ols: synthesis and evaluation of analgesic, anti-inflammatory and platelet anti-aggregating activities. Il Farmaco 2000, 55, 219–226. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Abdellatif, K.R.A.; Dong, Y.; Knaus, E.E. Synthesis of new 4-[2-(4-methyl(amino)sulfonylphenyl)-5-trifluoromethyl-2H-pyrazol-3-yl]-1,2,3,6-tetrahydropyridines: A search for novel nitric oxide donor anti-inflammatory agents. Bioorg. Med. Chem. 2008, 16, 8882–8888. [Google Scholar] [CrossRef] [PubMed]
- Banday, A.H.; Mir, B.P.; Lone, I.H.L.; Suri, K.A.; Kumar, H.M.S. Studies on novel D-ring substituted steroidal pyrazolines as potential anticancer agents. Steroids 2010, 75, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Asiri, A.M.; Khan, S.A. Synthesis and anti-bacterial activities of some novel schiff bases derived from aminophenazone. Molecules 2010, 15, 6850–6858. [Google Scholar] [CrossRef] [PubMed]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).