Abstract
A new Schiff base ester, 4-{[(4-fluorophenyl)imino]methyl}-3-hydroxyphenyl 4-(hexadecanoyloxy)benzoate was synthesized and its IR, 1H NMR, 13C NMR and EI-MS spectroscopic data are presented.
Schiff bases have attracted much attention from many researchers owing to their thermochromic and photochromic properties [1,2,3,4,5]. The presence of a long alkyl chain at the para position of the aldehyde or aniline fragment of N-benzylideneanilines has also been identified as one of the important requirements which favours the existence of liquid crystal phases [6,7,8]. Different alkyl chain length and terminal substituent can significantly influence the anisotropic properties of liquid crystals [6]. Thus, we report here another new derivative containing an hexadecanoyloxy chain, 4-{[(4-fluorophenyl)imino]methyl}-3-hydroxyphenyl 4-(hexadecanoyloxy)benzoate.
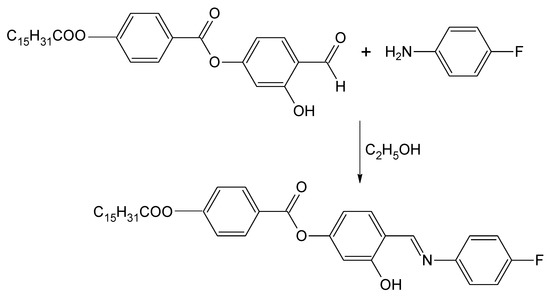
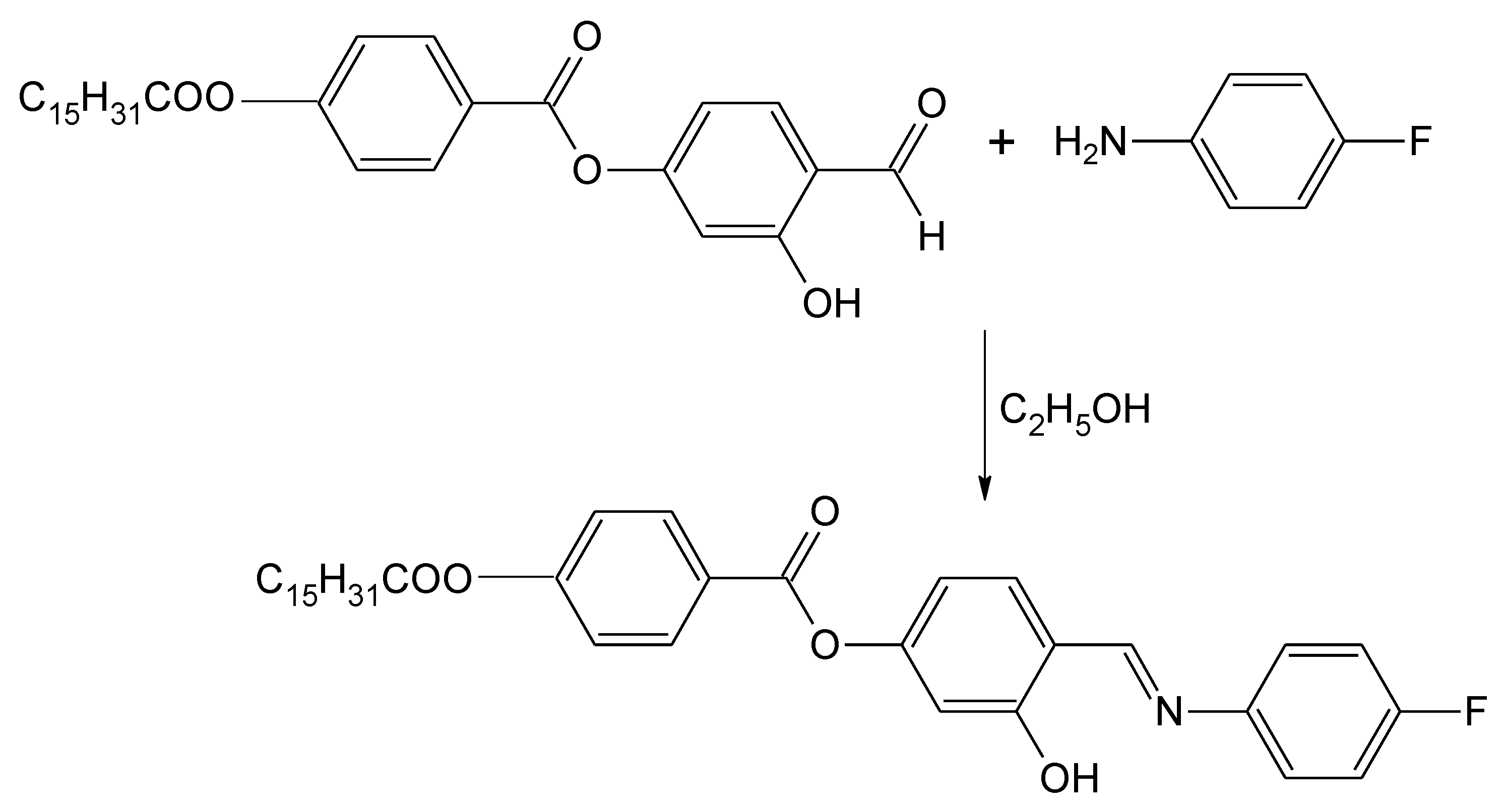
Scheme 1.
Synthesis of 4-{[(4-Fluorophenyl)imino]methyl}-3-hydroxyphenyl 4-(hexadecanoyloxy)benzoate.
Scheme 1.
Synthesis of 4-{[(4-Fluorophenyl)imino]methyl}-3-hydroxyphenyl 4-(hexadecanoyloxy)benzoate.
Experimental
Analytical data were obtained on Perkin Elmer 2400 LS series CHNS/O analyzers. Electron impact mass spectra (EI-MS) were recorded by Hewlett Packard 5989A Mass Spectrometer operating at 70 eV ionizing energy. FT-IR data were recorded on a Perkin Elmer 2000-FTIR spectrophotometer. NMR spectra were recorded in CDCl3 on a Bruker 400 MHz Ultrashield Spectrometer.
4-(4-n-Hexadecanoyloxybenzoyloxy)-2-hydroxybenzaldehye was prepared according to method described in our previous work [9]. In a round-bottom flask, a mixture of the aldehyde (2.48 g, 5.0 mmol), 4-fluoroaniline (0.56 g, 5.0 mmol) and absolute ethanol (40 mL) was refluxed with stirring for 3 h. The reaction mixture was filtered and the solvent was removed from the filtrate by evaporation. Recrystallization from absolute ethanol gave the title compound as a yellow solid (1.56 g, 53%).
Melting point: 189–191 °C
MS (EI): m/z (rel. int. %) = 590 (1) (M+).
IR (KBr): νmax (cm−1), 2953, 2916, 2848 (C-H aliphatic), 1755 (C=O of C15H31COO- fragment), 1743 (C=O of benzoate), 1625 (C=N), 1605 (C=C aromatic), 1282 (C-O).
1H NMR (400 MHz, CDCl3): δ/ppm, 0.91 (t, 3H, J = 6.8 Hz, CH3-), 1.24–1.47 (m, 24H, CH3-(CH2)12-), 1.79 (quint, 2H, J = 7.5 Hz, -CH2-CH2COO-), 2.62 (t, 2H, J = 7.5 Hz, -CH2-COO-), 6.86 (dd, 1H, J = 2.2, 8.4 Hz, Ar-H), 6.93 (d, 1H, J = 2.2 Hz, Ar-H), 7.12–7.17 (m, 2H, Ar-H), 7.26–7.31 (m, 4H, Ar-H), 7.46 (d, 1H, J = 8.5 Hz, Ar-H), 8.25 (d, 2H, J = 8.8 Hz, Ar-H), 8.62 (s, 1H, CH=N), 13.45 (s, 1H, OH).
13C NMR (100 MHz, CDCl3): δ/ppm, 171.86 (C=O of C15H31COO-), 164.11 (C=O of benzoate), 163.27 (C=N), 162.94, 161.94, 155.64, 155.14, 144.93, 133.54, 132.17, 127.08, 122.92, 122.25, 117.62, 116.49, 113.33 and 110.95 for aromatic carbons, 34.81 (-CH2COO-), 25.28 (-CH2CH2COO-), 32.27, 30.03, 30.02, 30.00, 29.98, 29.94, 29.79, 29.68, 29.58, 29.46, 23.01 (CH3(CH2)12), 14.37 (CH3(CH2)12).
Elemental analysis: Calculated for C36H44NO5F, 73.32%, H, 7.52%, N, 2.38%; Found: C, 73.37%, H, 7.50%, N, 2.40%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
Authors would like to thank Universiti Tunku Abdul Rahman and Universitit Sains Malaysia for the financial supports via UTAR Research Fund and research facilities.
References
- Hadjoudis, E.; Vittorakis, M. Moustakali-Mavridis, I. Photochromism and thermochromism of schiff bases in the solid state and in rigid glasses. Tetrahedron 1987, 43, 1345–1360. [Google Scholar] [CrossRef]
- Hadjoudis, E.; Rontoyianni, A.; Ambroziak, K.; Dziembowska, T.; Mavridis, I.M. Photochromism and thermochromism of solid trans-N,N'-bis(salicylidene)-1,2-cyclohexanediamines and trans-N,N'-bis-(2-hydroxynaphylidene)-1,2-cyclohexanediamine. J. Photochem. Photobiol. A Chem. 2004, 162, 521–530. [Google Scholar] [CrossRef]
- Oshima, A.; Momotake, A.; Arai, T. Photochromism, thermochromism, and solvatochromism of naphthalene-based analogues of salicylideneaniline in solution. J. Photochem. Photobiol. A Chem. 2004, 162, 473–479. [Google Scholar] [CrossRef]
- Yeap, G.Y.; Ha, S.T.; Ishizawa, N.; Suda, K.; Boey, P.L.; Mahmood, W.A.K. Synthesis, crystal structure and spectroscopic study of para substituted 2-hydroxy-3-methoxybenzalideneanilines. J. Mol. Struct. 2003, 658, 87–99. [Google Scholar] [CrossRef]
- Nair, S.M.; Bhattacharya, I. Synthesis and physiological activities of some imines and their β-lactams. Asian J. Chem. 2009, 21, 504–510. [Google Scholar]
- Yeap, G.Y.; Ha, S.T.; Lim, P.L.; Boey, P.L.; Ito, M.M.; Sanehisa, S.; Youhei, Y. Synthesis, physical and mesomorphic properties of Schiff’s base esters containing ortho-, meta- and para-substituents in benzylidene-4’-alkanoyloxyanilines. Liq. Cryst. 2006, 33, 205–211. [Google Scholar] [CrossRef]
- Ha, S.T.; Ong, L.K.; Wong, J.P.W.; Yeap, G.Y.; Lin, H.C.; Ong, S.T.; Koh, T.M. Mesogenic Schiff's base ether with dimethylamino end group. Phase Transit. 2009, 82, 387–397. [Google Scholar] [CrossRef]
- Ha, S.T.; Ong, L.K.; Ong, S.T.; Yeap, G.Y.; Wong, J.P.W.; Koh, T.M.; Lin, H.C. Synthesis and mesomorphic properties of new Schiff base esters with different alkyl chains. Chin. Chem. Lett. 2009, 20, 767–770. [Google Scholar] [CrossRef]
- Yeap, G.Y.; Ha, S.T.; Boey, P.L.; Mahmood, W.A.K.; Ito, M.M.; Youhei, Y. Synthesis and characterization of some new mesogenic Schiff base esters N-[4-(4-n-hexadecanoyloxybenzoyloxy)benzylidene]-4-substituted anilines. Mol. Cryst. Liq. Cryst. 2006, 452, 73–90. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
