Abstract
The title compound was synthesized by condensation of an oxiran imide derivative with an appropriate amine and its IR, 1H NMR, 13C NMR and mass spectroscopic data are reported. The synthesized compound was evaluated for its cytotoxicity and anti-HIV-1 activity in MT-4 cells.
Introduction
Currently available drugs for the treatment of HIV infections are based on combination of two types of anti-HIV-1 agents: nucleoside reverse transcriptase inhibitors (RTIs) and protease inhibitors [1]. The RTIs can be divided into nucleoside (NI) and non-nucleoside RT inhibitors (NNRTI). Several non-nucleoside inhibitors have been described, including nevirapine, thiobenzimidazolone (TIBO) derivatives, pyridinone derivatives and the bis(heteroaryl)piperazines (BHAPs), such as delavirdine and atevirdine [2]. Another arylpiperazine, vicriviroc, is currently under phase II of clinical investigation [3]. Therefore, the discovery of new BHAP analogs is actively pursued [4,5].
1-Acetyl-17-(oxiran-2-ylmethyl)-17-azapentacyclo[6.6.5.02,7.09,14.015,19]nonadeca-2,4,6,9,11,13-hexaene-16,18-dione 1 was used as a starting material. It was obtained from the reaction of imide 1-acetyl-17-azapentacyclo[6.6.5.02,7.09,14.015,19]nonadeca-2,4,6,9,11,13-hexaene-16,18-dione with 2-(chloromethyl)oxirane [6]. Next, it was subjected to the reaction with an amine to give the appropriate amino alcohol 2 (Scheme 1).
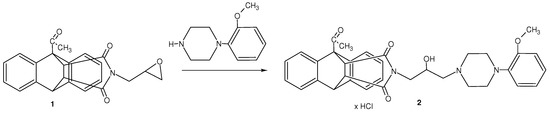
Scheme 1.
Synthesis of 4-amino-2-hydoxypropyl derivatives.
The synthesized compound was evaluated for its cytotoxicity and anti-HIV-1 activity in MT-4 cells [7]. The obtained compound 1 does not show activity against HIV-1. Compound concentation (µM) required to reduce the viability of mock-infected MT-4 cells by 50% is 45 (CC50). Compound concentration (µM) required to achieve 50% protection of MT-4 cells from the HIV-1 induced cytopathogeneticy is 45 or less (EC50).
Experimental Section
Chemistry
Synthesis of 1-acetyl-17-{2-hydroxy-3-[4-(2-methoxyphenyl)piperazin-1-yl]propyl}-17-azapentacyclo- [6.6.5.02,7.09,14.015,19]nonadeca-2,4,6,9,11,13-hexaene-16,18-dione hydrochloride (2)
A mixture of 1-acetyl-17-(oxiran-2-ylmethyl)-17-azapentacyclo[6.6.5.02,7.09,14.015,19]nonadeca-2,4,6,9,11,13-hexaene-16,18-dione (1) (0.5 g, 0.0013 mol) and (1-(2-methoxyphenyl)piperazine (0.43 g, 0.0019 mol in water/methanol (1:40) was refluxed for 50 h. The liquid was distilled off, the oily residue was purified by column chromatography (chloroform:methanol; 100:0.5 vol.). The dried residue was dissolved in methanol, and then 10 drops of HCl saturated methanol were added. The mixture was kept for 12 h at 6 °C and after that time the solvent was distilled off.
White crystals, yield 70%.
Melting point: 192 °C.
1H NMR (400 MHz, CDCl3) δ (ppm): 11.72 (s, 1H, NH+); 7.67–7.65 (m, 2H, CHarom.); 7.43–4.40 (m, 2H, CHarom.); 7.24–6.97 (m, 8H, CHarom.); 4.35–4.20 (m, 2H, CH); 4.02–3.87 (m, 2H, CH2); 3.96 (s, 3H, OCH3); 3.71–3.40 (m, 6H, CH2); 3.24–2.94 (m, 4H, CH2); 2.82 (d, 3H, CH3, J = 3.2 Hz).
13C-NMR (100 MHz, CDCl3) δ (ppm): 177.05, 177.02, 176.73, 141,52, 141,47, 140.14, 140.15, 138.62, 138.58, 137.27, 127.98, 127,78, 127.69, 127.59, 127.15, 125.51, 125.39, 125.32, 125.22, 124.74, 123.92, 123.32, 121.98, 64.03, 61.51, 60.77, 56.38, 50.95, 49.22, 48.57, 48.46, 46.20, 46.15, 42.5.
ESI MS: m/z = 566.12 [M]+ (100%).
Elemental Analysis: Calculated for C34H36ClN3O5 x 2H2O (638.15): C, 63.99%; H, 6.32%; N, 6.58%;. Found: C, 64.02%; H 6.30%; N, 6.55%.
Cells and Viruses
Cell lines were purchased from American Type Culture Collection (ATCC). The absence of mycoplasma contamination was checked periodically by the Hoechst staining method. Cell lines supporting the multiplication of RNA viruses were the following: CD4+ human T-cells containing an integrated HTLV-1 genome (MT-4).
Cytotoxicity Assays
For cytotoxicity evaluations, exponentially growing cells derived from human haematological tumors [CD4+ human T-cells containing an integrated HTLV-1 genome (MT-4)] were seeded at an initial density of 1 × 105 cells/mL in 96 well plates in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS), 100 units/mL penicillin G and 100 µg/mL streptomycin. Cell cultures were then incubated at 37 °C in a humidified, 5% CO2 atmosphere in the absence or presence of serial dilutions of test compounds. Cell viability was determined after 96 h at 37 °C by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) method [7].
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Carpenter, C.C.; Fischl, M.A.; Hammer, S.M.; Hirsch, M.S.; Jacobsen, D.M.; Katzenstein, D.A.; Montaner, J.S.; Richman, D.D.; Saag, M.S.; Schooley, R.T.; Thompson, M.A.; Vella, S.; Yeni, P.G.; Volberding, P.A. Antiretroviral therapy for HIV infection in 1998: Updated recommendations of the International AIDS Society-USA Panel. J. Am. Med. Assoc. 1998, 280, 78–86. [Google Scholar] [CrossRef]
- Romero, D.L.; Morge, R.A.; Genin, M.J.; Biles, C.; Busso, M.; Resnick, L.; Althaus, I.W.; Reusser, F.; Thomas, R.C.; Tarpley, W.G. Bis(heteroaryl)piperazine (BHAP) reverse transcriptase inhibitors: Structure-activity relationships of novel substituted indole analogues and the identification of 1-[(5-methanesulfonamido-1H-indol-2-yl)-carbonyl]-4-[3- [(1-methylethyl)amino]-pyridinyl]piperazine monomethanesulfonate (U-90152S), a second-generation clinical candidate. J. Med. Chem. 1993, 36, 1505–1508. [Google Scholar] [PubMed]
- Gulick, R.M.; Su, Z.; Flexner, C.; Hughes, M.D.; Skolnik, P.R.; Wilkin, T.J.; Gross, R.; Krambrink, A.; Coakley, E.; Greaves, W.L.; Zolopa, A.; Reichman, R.; Godfrey, C.; Hirsch, M.; Kuritzkes, D.R. Phase 2 study of the safety and efficacy of vicriviroc, a CCR5 inhibitor, in HIV-1-Infected, treatment-experienced patients: AIDS clinical trials group 5211. J. Infect. Dis. 2007, 196, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Pinna, G.A.; Loriga, G.; Murineddu, G.; Grella, G.; Mura, M.; Vargiu, L.; Murgioni, C.; La Colla, P. Synthesis and anti-HIV-1 activity of new delavirdine analogues carrying arylpyrrole moieties. Chem. Pharm. Bull. 2001, 49, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Tagat, J.R.; McCombie, S.W.; Nazareno, D.; Labroli, M.A.; Xiao, Y.; Steensma, R.W.; Strizki, J.M.; Baroudy, B.M.; Cox, K.; Lachowicz, J.; Varty, G.; Watkins, R. Piperazine-based CCR5 antagonists as HIV-1 inhibitors. IV. Discovery of 1-[(4,6-dimethyl-5-pyrimidinyl)carbonyl]-4-[4-[2-methoxy-1(R)-4-(trifluoromethyl)phenyl]ethyl-3(S)-methyl-1-piperazinyl]-4-methylpiperidine (Sch-417690/Sch-D), a potent, highly selective, and orally bioavailable CCR5 antagonist. J. Med. Chem. 2004, 47, 2405–2408. [Google Scholar]
- Kossakowski, J.; Szczepek, W.; Pakosińska-Parys, M. Synthesis of new n-substituted cyclic imides with expected anxiolytic activity. XXI. Derivatives of 1-acetyldibenzo-[e.h]-bicyclo[2.2.2]-octane-2,3-dicarboximide. Acta Pol. Pharm. 2002, 59, 418–423. [Google Scholar] [PubMed]
- Pauwels, R.; Balzarini, J.; Baba, M.; Snoeck, R.; Schols, D.; Herdewijn, P.; Desmyster, J.; De Clercq, E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 1998, 20, 309–321. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).