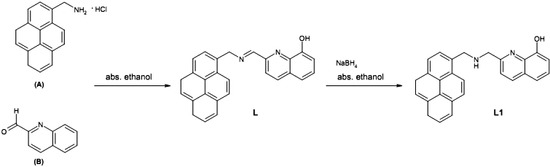
2-((Pyren-1-ylmethylamino)methyl)quinolin-8-ol
Abstract
:1. Introduction
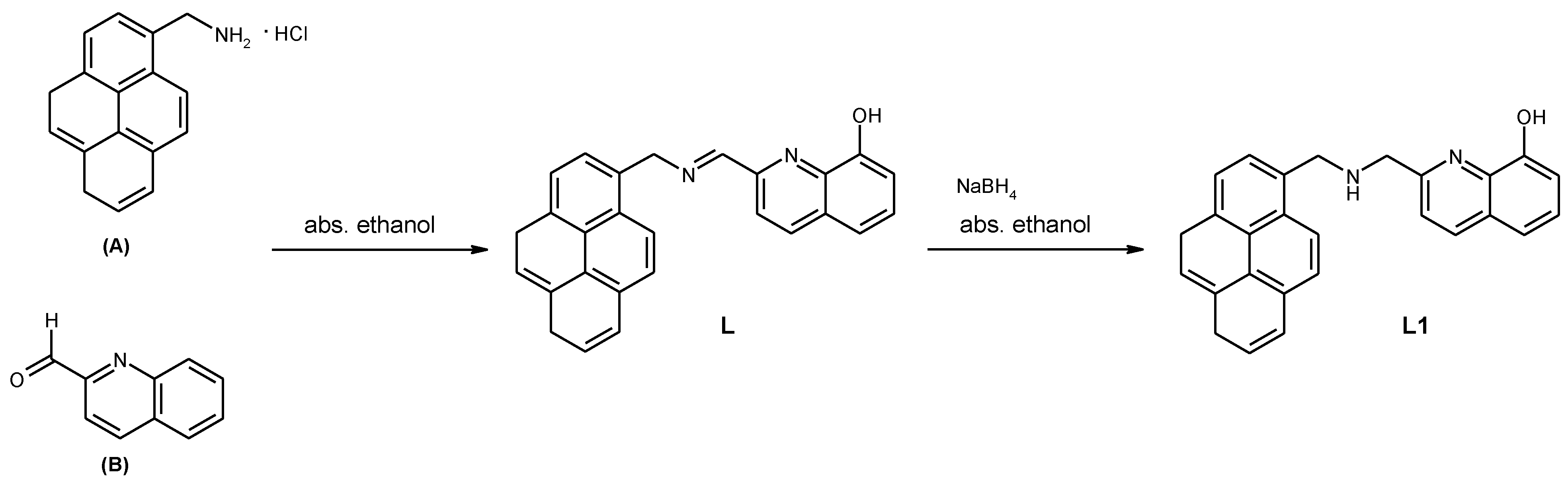
2. Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Lodeiro, C.; Pina, F. Luminescent and chromogenic molecular probes based on polyamines and related compounds. Coord. Chem. Rev. 2009, 253, 1353–1385. [Google Scholar] [CrossRef]
- Lodeiro, C.; Capelo, J.L.; Mejuto, J.C.; Oliveira, E.; Santos, H.M.; Pedras, B.; Núñez, C. Light and colour as analytical detection tools: A journey into the periodic table using polyamines to bio-inspired systems as chemosensors. Chem. Soc. Rev. 2010, 39, 2948–2976. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef] [PubMed]
- De Armas, G.; Miro, M.; Cladera, A.; Estela, J.M.; Cerda, V. Time-based multisyringe flow injection system for the spectrofluorimetric determination of aluminium. Anal. Chim. Acta 2002, 455, 149–157. [Google Scholar] [CrossRef]
- Bronson, R.T.; Montalti, M.; Prodi, L.; Zaccheroni, N.; Lamb, R.D.; Dalley, N.K.; Izatt, R.M.; Bradshaw, J.S.; Savage, P.B. Origins of ‘on–off’ fluorescent behavior of 8-hydroxyquinoline containing chemosensors. Tetrahedron 2004, 60, 11139–11144. [Google Scholar] [CrossRef]
- Wang, S. Luminescence and electroluminescence of Al(III), B(III), Be(II) and Zn(II) complexes with nitrogen donors. Coord. Chem. Rev. 2001, 215, 79–98. [Google Scholar] [CrossRef]
- Muegge, B.D.; Brooks, S.; Richter, M.M. Electrochemiluminescence of tris(8-hydroxyquinoline-5-sulfonic acid)aluminum(III) in aqueous solution. Anal. Chem. 2003, 75, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Karpovich, D.S.; Blanchard, G.J. Relating the polariry dependent fluorescence response of pyrene to vibronic coupling-achieving a fundamental understanding of the py polariry scale. J. Phys. Chem. 1995, 99, 3951–3958. [Google Scholar] [CrossRef]
- Birks, J.B. Photophysics of Aromatic Molecules; Wiley: London, UK, 1970. [Google Scholar]
- Lodeiro, C.; Lima, J.C.; Parola, A.J.; Seixas de Melo, J.S.; Capelo, J.L.; Covelo, B.; Tamayo, A.; Pedras, B. Intramolecular eximer formation and sensing behavior of new fluorimetric probes and their interactions with metal cations and barbituric acids. Sensor. Actuator B-Chem. 2006, 115, 276–286. [Google Scholar] [CrossRef]
- Corma, A.; Galletero, M.S.; García, H.; Palomares, E.; Rey, F. Pyrene covalently anchored on a large external surface are zeolite as a selective heterogeneous sensor for iodide. Chem. Commun. 2002, 1100–1101. [Google Scholar] [CrossRef]
- Seixas de Melo, J.; Costa, T.; Miguel, M.D.G.; Lindman, B.; Schillén, K. Time-resolved and steady-state fluorescence studies of hydrophobically modified water-soluble polymers. J. Phys. Chem. B 2003, 107, 12605–12621. [Google Scholar] [CrossRef]
- Sasaki, D.; Padilla, B.E. Dithioamide metal ion receptors on fluorescent lipid bilayers for the selective optical detection of mercuric ion. Chem. Commun. 1998, 1581–1582. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fernández-Lodeiro, J.; Nuñez, C.; Capelo, J.L.; Lodeiro, C. 2-((Pyren-1-ylmethylamino)methyl)quinolin-8-ol. Molbank 2010, 2010, M698. https://doi.org/10.3390/M698
Fernández-Lodeiro J, Nuñez C, Capelo JL, Lodeiro C. 2-((Pyren-1-ylmethylamino)methyl)quinolin-8-ol. Molbank. 2010; 2010(4):M698. https://doi.org/10.3390/M698
Chicago/Turabian StyleFernández-Lodeiro, Javier, Cristina Nuñez, José Luis Capelo, and Carlos Lodeiro. 2010. "2-((Pyren-1-ylmethylamino)methyl)quinolin-8-ol" Molbank 2010, no. 4: M698. https://doi.org/10.3390/M698
APA StyleFernández-Lodeiro, J., Nuñez, C., Capelo, J. L., & Lodeiro, C. (2010). 2-((Pyren-1-ylmethylamino)methyl)quinolin-8-ol. Molbank, 2010(4), M698. https://doi.org/10.3390/M698