Abstract
Trans-dichloro-2,3-naphthalenediamine bis[(2-methoxyethyl)(diphenyl)phos-phine]ruthenium(II) complex Cl2Ru(η1-Ph2PCH2CH2OCH3)2(C10H10N2) has been obtained by reaction of equimolar amounts of Cl2Ru(P⌒O)2 complex 2 with one equivalent of 2,3-naphthalenediamine as co-ligand in very good yield. The structure of this new complex 3 was confirmed by elemental analysis, IR, 31P-NMR 1H-NMR, 13C-NMR, UV-visible spectroscopy and FAB-MS.
1. Introduction
Bifunctional ether-phosphines (O,P) have significantly affected the isolation of coordinatively unsaturated species [1,2,3,4,5,6,7]. These ligands are provided with oxygen atoms incorporated in open-chain ether moieties which form a weak metal-oxygen contact while the phosphorus atom is strongly coordinated to the metal [3,4,5,6,7,8,9]. In these “hemilabile“ ligands, the ether moiety is regarded as an intramolecular solvent molecule stabilizing the vacant coordination site by chelation. Phosphorus–oxygen hemilabile ligands like 2-(diphenylphosphino)ethyl methyl ether (P~O), reacts with various metals of catalytic relevance due to their ability to act as both a chelate ligand, stabilizing the metal complex, and a monodentate ligand providing a free coordination site for an incoming substrate (through the labilization of the weakly bonded oxygen atom) [1,2,3,4,5,6,7,8,9,10].
2. Result and Discussion
The Ph2PCH2CH2OCH3 ligand and complex 2 were synthesized according to literature [2]. Treating complex 2 with an equivalent amount of 2,3-naphthalenediamine as co-ligand in dichloromethane at room temperature resulted in the formation of complex 3 without any side products as shown in Scheme 1.
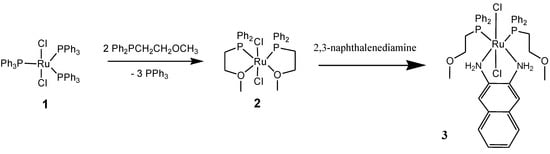
Scheme 1.
The synthetic route to complex 3.
The stepwise formation of the desired complex 3 is monitored by 31P{1H}-NMR spectroscopy, in an NMR tube experiment, where addition of 2,3-naphthalenediamine to a CD2Cl2 solution containing Cl2Ru(P⌒O)2 complex as starting material leads to the disappearance of the red color of the Cl2Ru(P⌒O)2 complex and the singlet of this complex at δp = 64.2 ppm and the appearance of the singlet at δp = 41.3 ppm due to the formation of complex 3 with a trans-Cl2Ru(P~O)(N⌒N) formula as shown in Figure 1.
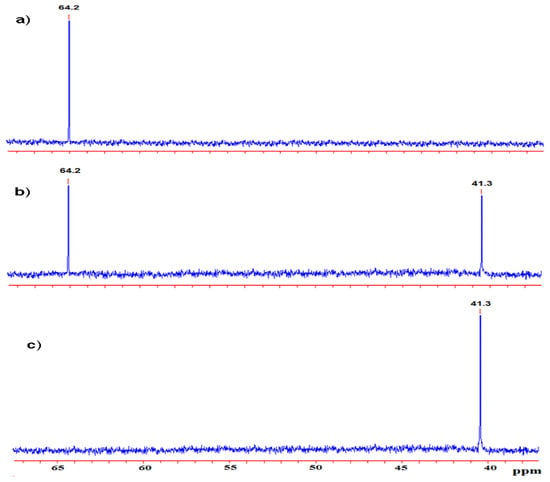
Figure 1.
Time-dependent 31P{1H}-NMR spectroscopy of complex 2 at δp = 64.2 ppm mixed with 1 equivalent of 2,3-naphthalenediamine co-ligand in CD2Cl2 in the NMR tube to produce complex 3 at δp = 41.3 ppm a) before co-ligand addition, b) the first shot ~ 40 second and c) the second shot ~1 min. after the co-ligand addition.
Liquid 31P{1H}-NMR spectra using CD2Cl2 show that complex 3 formed as trans-Cl2Ru(P~O)(N⌒N), since only a singlet at δp = 41.3 ppm is detected without any other singlets. If cis-Cl2Ru(P~O)(N⌒N isomer was formed, an AB 31P{1H}-NMR pattern with a JPP coupling constant of ~40 to 100 Hz would be detected due to the formation of inequivalent phosphorus atoms.
3. Experimental
2,3-Naphthalenediamine (0.04 g, 0.25 mmol) was dissolved in 10 mL of dichloromethane and the solution was added dropwise to a stirred solution of Cl2Ru(P⌒O)2 (0.17 g, 0.25 mmol) in 15 mL of dichloromethane. After the reaction mixture was stirred for approximately 20 min at room temperature, the solution was concentrated to a volume of ~1 mL under reduced pressure. Addition of 30 mL of diethyl ether caused the precipitation of a solid which was filtered (P4), washed well with 25 mL of n-hexane and dried under vacuum.
Melting point: 280 °C
Yield: 89% (0.18 g) of a violet powder.
MS (FAB): m/z = 818.2 (M+).
IR (KBr, cm-1): 3340 (vNH), 3180 (vPhH) and 2970 (vCH). 1540 (vC=C).
UV-visible absorption: λmax = 270 nm and 503 nm.
31P{1H} NMR (CDCl3): δ (ppm) 41.3.
1H NMR (CDCl3): δ (ppm) 2.4 (m, 4H, PCH2), 2.9 (s, 6H, OCH3), 3.0 (m, 4H, OCH2), 4.4 (b, 4H, NH2), 6.6–7.7 (4m, 26H, Ph).
13C{1H} NMR (CDCl3): δ (ppm) 24.7 (m, 2C, PCH2), 57.6 (s, 2C, OCH3), 68.6 (s, 2C, OCH2), 126.8–132.4 (9s, 34C, Phs).
Elemental analysis: Calcd for C40H44Cl2N2O2P2Ru. C, 58.68; H, 5.42; Cl, 8.66; N, 3.42%. Found: C, 58.23; H, 5.77; Cl, 8.28; N, 3.32%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
I would like to thank the Research Center/Science College / King Saud University for financial support.
References and Notes
- Warad, I. Supported and Non-Supported Ruthenium(II)/Phosphine/[3-(2-Aminoethyl)aminopropyl]trimethoxysilane Complexes and Their Activities in the Chemoselective Hydrogenation of trans-4-Phenyl-3-butene-2-al. Molecules 2010, 15, 4652–4669. [Google Scholar] [CrossRef] [PubMed]
- Lindner, E.; Warad, I.; Eichele, K.; Mayer, H.A. Synthesis and Structures of an Array of Diamine(ether-phosphine)ruthenium(II) Complexes and Their Application in the Catalytic Hydrogenation of trans-4-phenyl-3-butene-2-one. Inorg. Chim. Acta 2003, 350, 49–56. [Google Scholar] [CrossRef]
- Lu, Z.-L.; Eichele, K.; Warad, I.; Mayer, H.A.; Lindner, E.; Jiang, Z.; Schurig, V. Supported Organometallic Complexes. XXXVIII Bis(methoxyethyldimethylphosphine)ruthenium(II) Complexes as Transfer Hydrogenation Catalysts. Z. Anorg. Allg. Chem. 2003, 629, 1308–1315. [Google Scholar] [CrossRef]
- Warad, I.; Lindner, E.; Eichele, K.; Mayer, A.H. Cationic Diamine(ether–phosphine)ruthenium(II) Complexes as Precursors for the Hydrogenation of trans-4-phenyl-3-butene-2-one. Inorg. Chim. Acta 2004, 357, 1847–1853. [Google Scholar] [CrossRef]
- Lindner, E.; Ghanem, A.; Warad, I.; Eichele, K.; Mayer, H.A.; Schurig, V. Asymmetric Hydrogenation of an Unsaturated Ketone by Diamine(ether–phosphine)ruthenium(II) Complexes and Lipase-Catalyzed Kinetic Resolution: A Consecutive Approach. Tetrahedron Asymmetry 2003, 14, 1045–1050. [Google Scholar]
- Lindner, E.; Al-Gharabli, S.; Warad, I.; Mayer, H.A.; Steinbrecher, S.; Plies, E.; Seiler, M.; Bertagnolli, H. Diaminediphosphineruthenium(II) Interphase Catalysts for the Hydrogenation of α,ß-Unsaturated Ketones. Z. Anorg. Allg. Chem. 2003, 629, 161–171. [Google Scholar] [CrossRef]
- Lu, Z.-L.; Eichele, K.; Warad, I.; Mayer, H.A.; Lindner, E.; Jiang, Z.; Schurig, V. Bis(methoxyethyldimethylphosphine)ruthenium(II) Complexes as Transfer Hydrogenation Catalysts. Z. Anorg. Allg. Chem. 2003, 629, 1308–1315. [Google Scholar] [CrossRef]
- Warad, I.; Al-Resayes, S.; Eichele, E. Crystal Structure of trans-Dichloro-1,3-propanediamine-bis-[(2-methoxyethyl)diphenylphosphine]ruthenium(II), RuCl2(C3H10N2)(C15H17OP)2. Z. Kristallogr. NCS 2006, 221, 275–277. [Google Scholar]
- Warad, I. Synthesis and Crystal Structure of cis-Dichloro-1,2-ethylenediamine-bis[1,4-(diphenylphosphino)butane]ruthenium(II) Dichloromethane Disolvate, RuCl2(C2H8N2)(C28H28P2)-2CH2Cl2. Z. Kristallogr. NCS 2007, 222, 415–417. [Google Scholar]
- Warad, I.; Siddiqui, M.; Al-Resayes, S.; Al-Warthan, A.; Mahfouz, R. Synthesis, Characterization, Crystal Structure and Chemical Behavior of [1,1-Bis(diphenylphosphinomethyl)ethene]ruthenium (II) Complex Toward Primary Alkylamine Addition. Trans. Met. Chem. 2009, 34, 347–354. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).