Abstract
The title compound, N-[(9-ethyl-9H-carbazol-3-yl)methylene]-3,4-dimethyl-isoxazol-5-amine has been synthesized by reaction of 9-ethyl-9H-carbazole-3-carbaldehyde with 5-amino-3,4-dimethylisoxazole in the presence of acetic acid in ethanol. The structure of this new compound was confirmed by elemental analysis, IR, 1H-NMR, 13C-NMR and EI-MS spectral analysis.
Schiff base compounds are generally known due to the azomethine group present. These compounds are usually synthesized by condensation of primary amines and active carbonyl groups. Schiff bases are an important class of compounds in the medicinal and pharmaceutical field. They show biological applications including antibacterial [], antifungal [], anticancer [], anti-inflammatory [] and antitumor activity []. Heterocycle-containing derivatives of Schiff bases are known to possess a variety of biological activities such as CNS depressant, anticancer [], antibiotic [], antihistaminic [], anticonvulsant [] and many others. Due to the wide application of hetrocyclic Schiff bases, we undertook the synthesis of a new heterocyclic Schiff base from a carbazole aldehyde and 5-amino-3,4-dimethylisoxazole.
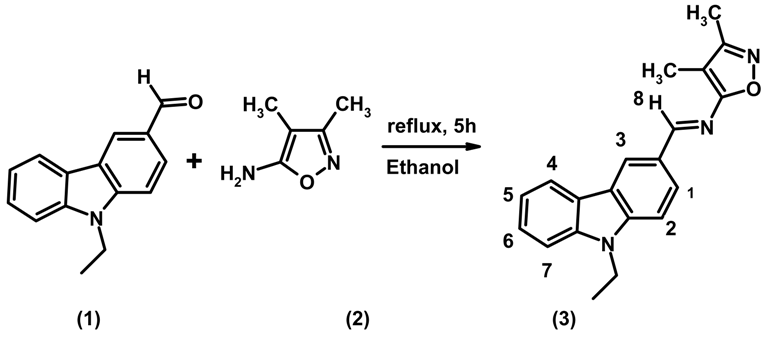
Experimental
A mixture of 9-ethyl-9H-carbazole-3-carbaldehyde (0.50 g, 0.0022 mol) and 5-amino-3,4-dimethyl-isoxazole (0.36 g, 0.0022 mol) in ethanol (15 mL) was refluxed for 5 h with stirring to give a light yellow precipitate. This material was filtered off and washed with ethanol to give the pure Schiff base. Figure 1a and Figure 1b showed the absorption and emission spectra of compound 3 in chloroform solution.
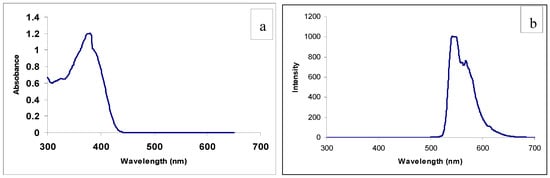
Figure 1.
(a) UV-Visible absorption of compound 3; (b) Emission Spectrum of compound 3.
Yield: 88%; mp: 188–189 οC
EI-MS m/z (rel. int.%): 318 (68) [M + 1]+
IR (KBr) vmax cm-1: 2971 (C-H), 1627 (C=C), 1584 (HC=N), 1126 (C-N).
¹H NMR (CDCl3) δ: 8.16 (d. H1, J = 7.8 Hz), 8.09 (d, H2, J = 8.4 Hz), 8.63 (s, H3), 7.32 (d, H4, J = 7.2 Hz), 7.53 (dd, H5, J = 7.8 Hz), 7.45 (dd, H6, J = 4.8 Hz), 7.29 (d, H7, J = 7.4 Hz), 9.00 (s, H8, CH olefinic), 4.40 (t, N-CH2-CH3, J = 7.2 Hz), 1.48 (q, N-CH2-CH3, J = 8.4 Hz), 2.26 (s, CH3), 2.12 (s, CH3).
Anal. calc. for C20H19N3O: C, 75.69, H, 6.03, N, 13.24. Found: C, 75.66, H, 5.98, N, 13.21.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Rosu, T.; Pahontu, E.; Maxim, C.; Georgescu, R.; Stanica, N.; Almajan, G.L.; Gulea, A. Synthesis, characterization and antibacterial activity of some new complexes of Cu(II), Ni(II), VO(II), Mn(II) with Schiff base derived from 4-amino-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one. Polyhedron 2010, 29, 757–766. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Sumrra, H.S.; Youssoufi, M.H.; Hadda, T.B. Metal based biologically active compounds: Design, synthesis, and antibacterial/antifungal/cytotoxic properties of triazole-derived Schiff bases and their oxovanadium(IV) complexes. Eur. J. Med. Chem. 2010, 45, 2739–2747. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, B.; Basu, S.; Chakraborty, P.; Choudhuri, S.K.; Mukherjee, A.K.; Mukherjee, M. Synthesis, spectroscopic characterization, X-ray powder structure analysis, DFT study and in vitro anticancer activity of N-(2-methoxyphenyl)-3-methoxysalicylaldimine. J. Mol. Struct. 2009, 932, 90–96. [Google Scholar] [CrossRef]
- Cardile, V.; Panico, A.M.; Geronikaki, A.; Gentile, B.; Ronsisvalle, G. In vitro evaluation of thiazolyl and benzothiazolyl Schiff bases on pig cartilage. II Farmaco 2002, 57, 1009–1013. [Google Scholar] [CrossRef]
- Zhong, X.; Wei, H.; Liu, W.; Wang, D.; Wang, X. The crystal structures of copper(II), manganese(II), and nickel(II) complexes of a (Z)-2-hydroxy-N′-(2-oxoindolin-3-ylidene) benzohydrazide—potential antitumor agents. Bioorg. Med. Chem. Lett. 2007, 17, 3774–3777. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, Y.; Momekov, G.; Petrov, O.; Karaivanova, M.; Kalcheva, V. Cytotoxic Mannich bases of 6-(3-aryl-2-propenoyl)-2(3H)-benzoxazolones. Eur. J. Med. Chem. 2007, 42, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Krammer, B.; Kader, N.A.; Verwanger, T.; El-Ansary, A. Antibacterial effect of some benzopyrone derivatives. Eur. J. Med. Chem. 2010, 45, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Metri, R.M.; Kulkarni, V.H.; Patil, S.A. Synthesis and antihistaminic activity of oxovanadium(IV) complexes of some new Schiff bases. J. Inorg. Biochem. 1995, 59, 604. [Google Scholar] [CrossRef]
- Viossat, B.; Daran, J.; Savouret, G.; Morgant, G.; Greenaway, F.T.; Dung, N.; Pham-Tran, V.A.; Sorenson, R.J. Low-temperature (180 K) crystal structure, electron paramagnetic resonance spectroscopy, and propitious anticonvulsant activities of CuII2(aspirinate)4(DMF)2 and other CuII2(aspirinate)4 chelates. J. Inorg. Biochem. 2003, 96, 375–385. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).