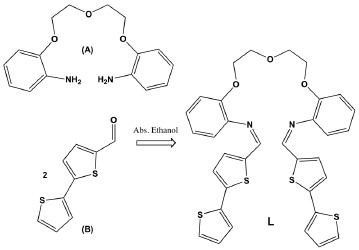
2,2'-(2,2'-Oxybis(ethane-2,1-diyl)bis(oxy))bis(N-(2,2'-bithiophen-5-ylmethylene)aniline)
Abstract
:1. Introduction
2. Experimental

Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
References
- Czarnik, A.W. Chemical Communication in water using Fluorescent chemosensors. Acc. Chem. Res. 1994, 27, 302–308. [Google Scholar] [CrossRef]
- Lodeiro, C.; Pina, F. Luminescent and chromogenic molecular probes based on polyamines and related compounds. Coord. Chem. Rev. 2009, 253, 1353–1385. [Google Scholar] [CrossRef]
- Cozzi, P.G.; Dolci, L.C.; Garelli, A.; Montati, M.; Prodi, L.; Zaccheroni, N. Photophysical properties of Schiff-base metal complexes. New J. Chem. 2003, 27, 692–697. [Google Scholar] [CrossRef]
- Si, S.H.; Chen, F.R.; Zhou, Y.F.; Wang, J.N.; Zhang, H.; Xu, J.G. Enhanced fluorescence sensing of hydroxylated organotins by a boronic acid-linke Schiff-base. Chem. Commun. 2009, 4179–4181. [Google Scholar]
- Vázquez, M.; Bermejo, R.M.; Sanmartín, J.; García-Deibe, A.M.; Lodeiro, C.; Mahía, J. Metal complexes with a chiral N4 symmetrical Schiff base. Crystal structures of the ligand and its Cu(II) and Ni(II) “mono-helicates”. J. Chem. Soc. Dalton Trans. 2002, 870–877. [Google Scholar] [CrossRef]
- Lodeiro, C.; Capelo, J.L. Metal ion interaction of two new tritopic N2O3 macrobicycles with B15C5 moieties in acetonitrile solution. J. Inclu. Phen. Mac. Chem. 2004, 49, 249–258. [Google Scholar] [CrossRef]
- Lodeiro, C.; Lima, L.C.; Parola, A.J.; Seixas de Melo, J.S.; Capelo, J.L.; Covelo, B.; Tamayo, A.; Pedras, B. Intramolecular excimer formation and sensing behaviour of new fluorimetric probes and their interactions with metal cations and barbituric acids. Sensors Act. B Chem. 2006, 115, 276–286. [Google Scholar] [CrossRef]
- Pedras, B.; Santos, H.M.; Fernandes, L.; Covelo, B.; Tamayo, A.; Bértolo, E.; Capelo, J.L.; Avilés, T.; Lodeiro, C. Sensing metal ions with two azomethine-thiophene pincer ligands (NSN): Fluorescence and MALDI-TOF-MS applications. Inorg. Chem. Commun. 2007, 10, 925–929. [Google Scholar] [CrossRef]
- Fernandes, L.; Boucher, M.; Fernández-Lodeiro, J.; Oliveira, E.; Nuñez, C.; Santos, H.M.; Capelo, J.L.; Nieto-Faza, O.; Bértolo, E.; Lodeiro, C. Exploiting anionic and cationic interactions with a new emissive imine-based b-naphthol molecular probe. Inorg. Chem. Commun. 2009, 12, 925–912. [Google Scholar] [CrossRef]
- Pedras, B.; Fernandes, L.; Oliveira, E.; Rodríguez, L.; Raposo, M.M.M.; Capelo, J.L.; Lodeiro, C. Synthesis, characterization and spectroscopic studies of two new Schiff-base bithienyl pendant-armed 15-crown-5 molecular probes. Inorg. Chem. Commun. 2009, 12, 79–85. [Google Scholar] [CrossRef]
- Pedras, B.; Oliveira, E.; Santos, H.; Rodríguez, L.; Crehuet, L.; Avilés, T.; Capelo, J.L.; Lodeiro, C. A new tripodal poly-imine indole-containing ligand: Synthesis, complexation, spectroscopic and theoretical studies. Inorg. Chim. Acta 2009, 362, 2627–2635. [Google Scholar] [CrossRef]
- Lodeiro, C.; Capelo, J.L. Beyond the Analytical Chemistry. In Ultrasound in Chemistry. Analytical Applications; Capelo, J.L., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 129–148. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pedras, B.; Gallego, D.; Figueiredo, P.; Nunes-Miranda, J.D.; Capelo, J.L.; Lodeiro, C. 2,2'-(2,2'-Oxybis(ethane-2,1-diyl)bis(oxy))bis(N-(2,2'-bithiophen-5-ylmethylene)aniline). Molbank 2010, 2010, M683. https://doi.org/10.3390/M683
Pedras B, Gallego D, Figueiredo P, Nunes-Miranda JD, Capelo JL, Lodeiro C. 2,2'-(2,2'-Oxybis(ethane-2,1-diyl)bis(oxy))bis(N-(2,2'-bithiophen-5-ylmethylene)aniline). Molbank. 2010; 2010(2):M683. https://doi.org/10.3390/M683
Chicago/Turabian StylePedras, Bruno, Daniel Gallego, Patrick Figueiredo, Júlio Dinis Nunes-Miranda, José Luis Capelo, and Carlos Lodeiro. 2010. "2,2'-(2,2'-Oxybis(ethane-2,1-diyl)bis(oxy))bis(N-(2,2'-bithiophen-5-ylmethylene)aniline)" Molbank 2010, no. 2: M683. https://doi.org/10.3390/M683
APA StylePedras, B., Gallego, D., Figueiredo, P., Nunes-Miranda, J. D., Capelo, J. L., & Lodeiro, C. (2010). 2,2'-(2,2'-Oxybis(ethane-2,1-diyl)bis(oxy))bis(N-(2,2'-bithiophen-5-ylmethylene)aniline). Molbank, 2010(2), M683. https://doi.org/10.3390/M683